c-Jun-deficient cells undergo premature senescence as a result of spontaneous DNA damage accumulation
- PMID: 15456874
- PMCID: PMC517871
- DOI: 10.1128/MCB.24.20.9006-9018.2004
c-Jun-deficient cells undergo premature senescence as a result of spontaneous DNA damage accumulation
Abstract
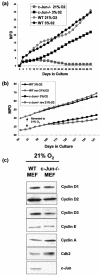
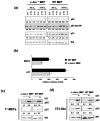
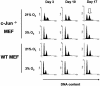
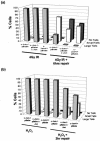
Mouse embryo fibroblasts deficient for the c-Jun proto-oncogene (c-Jun-/- MEF) undergo p53-dependent premature senescence in conventional culture. This phenotype becomes evident only after several cell divisions, suggesting that senescence may result from exposure to unknown environmental factors. Here, we show that c-Jun-/- MEF can proliferate successfully in low oxygen (3% O2), indicating that premature senescence under conventional culture conditions is a consequence of hyperoxic stress. c-Jun-/- MEF exhibit higher basal levels of DNA damage compared to normal fibroblasts in high but not low oxygen, implying that senescence results from chronic accumulation of spontaneous DNA damage. This accumulation may be attributable, at least in part, to inefficient repair, since DNA damage induced by gamma ionizing radiation and H2O2 persists for longer in c-Jun-/- MEF than in wild-type MEF. Unexpectedly, p53 expression, phosphorylation, and transcriptional activity are largely unaffected by oxygen exposure, indicating that the accumulation of spontaneous DNA damage does not result in chronic activation of p53 as judged by conventional criteria. Finally, we find that c-Jun associates with nuclear foci containing gammaH2AX and ATM following irradiation, suggesting a potential role for c-Jun in DNA repair processes per se.
Figures





Similar articles
-
ATM-dependent telomere loss in aging human diploid fibroblasts and DNA damage lead to the post-translational activation of p53 protein involving poly(ADP-ribose) polymerase.EMBO J. 1997 Oct 1;16(19):6018-33. doi: 10.1093/emboj/16.19.6018. EMBO J. 1997. PMID: 9312059 Free PMC article.
-
Induction of ATF3 by ionizing radiation is mediated via a signaling pathway that includes ATM, Nibrin1, stress-induced MAPkinases and ATF-2.Oncogene. 2003 Jul 3;22(27):4235-42. doi: 10.1038/sj.onc.1206611. Oncogene. 2003. PMID: 12833146
-
Proinflammatory cytokine-induced cellular senescence of biliary epithelial cells is mediated via oxidative stress and activation of ATM pathway: a culture study.Free Radic Res. 2008 Jul;42(7):625-32. doi: 10.1080/10715760802244768. Epub 2008 Jun 25. Free Radic Res. 2008. PMID: 18608517
-
Replicative senescence and oxidant-induced premature senescence. Beyond the control of cell cycle checkpoints.Ann N Y Acad Sci. 2000 Jun;908:111-25. doi: 10.1111/j.1749-6632.2000.tb06640.x. Ann N Y Acad Sci. 2000. PMID: 10911952 Review.
-
Jun dimerization protein 2 in oxygen restriction; control of senescence.Curr Pharm Des. 2011;17(22):2278-89. doi: 10.2174/138161211797052394. Curr Pharm Des. 2011. PMID: 21736542 Review.
Cited by
-
In situ proximity ligation detection of c-Jun/AP-1 dimers reveals increased levels of c-Jun/Fra1 complexes in aggressive breast cancer cell lines in vitro and in vivo.Mol Cell Proteomics. 2010 Sep;9(9):1982-90. doi: 10.1074/mcp.M110.000943. Epub 2010 May 28. Mol Cell Proteomics. 2010. PMID: 20511396 Free PMC article.
-
Linking JNK Activity to the DNA Damage Response.Genes Cancer. 2013 Sep;4(9-10):360-8. doi: 10.1177/1947601913486347. Genes Cancer. 2013. PMID: 24349633 Free PMC article. Review.
-
Tfcp2l1 as a central integrator of hypoxia, dedifferentiation, and tumor progression.J Exp Clin Cancer Res. 2025 Aug 14;44(1):236. doi: 10.1186/s13046-025-03501-9. J Exp Clin Cancer Res. 2025. PMID: 40813991 Free PMC article. Review.
-
The Role of Activator Protein-1 (AP-1) Family Members in CD30-Positive Lymphomas.Cancers (Basel). 2018 Mar 28;10(4):93. doi: 10.3390/cancers10040093. Cancers (Basel). 2018. PMID: 29597249 Free PMC article. Review.
-
FoxM1 regulates transcription of JNK1 to promote the G1/S transition and tumor cell invasiveness.J Biol Chem. 2008 Jul 25;283(30):20770-8. doi: 10.1074/jbc.M709892200. Epub 2008 Jun 4. J Biol Chem. 2008. PMID: 18524773 Free PMC article.
References
-
- Black, E. J., W. Clark, and D. A. Gillespie. 2000. Transient deactivation of ERK signalling is sufficient for stable entry into G0 in primary avian fibroblasts. Curr. Biol. 10:1119-1122. - PubMed
-
- Burma, S., B. P. Chen, M. Murphy, A. Kurimasa, and D. J. Chen. 2001. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 276:42462-42467. - PubMed
-
- Chiang, Y. C., S. C. Teng, Y. N. Su, F. J. Hsieh, and K. J. Wu. 2003. c-Myc directly regulates the transcription of the NBS1 gene involved in DNA double-strand break repair. J. Biol. Chem. 278:19286-19291. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
