Genetically increasing Myoc expression supports a necessary pathologic role of abnormal proteins in glaucoma
- PMID: 15456875
- PMCID: PMC517885
- DOI: 10.1128/MCB.24.20.9019-9025.2004
Genetically increasing Myoc expression supports a necessary pathologic role of abnormal proteins in glaucoma
Abstract
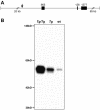
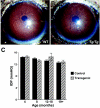
Despite the importance of MYOC for glaucoma, the protein's normal function(s) and the pathogenic mechanism(s) of MYOC mutations are not clear. Elevated intraocular pressure (IOP) and glaucoma are sometimes induced by corticosteroids, and corticosteroid use can result in substantially increased MYOC expression. It has been suggested, therefore, that steroid-induced MYOC protein levels cause steroid-induced glaucoma and that protein level-increasing mutations in MYOC contribute to glaucoma not associated with steroid use. A causative role of elevated MYOC levels in steroid-induced glaucoma is controversial, however, and it is not clear if elevated MYOC levels can result in IOP elevation. To directly test if increased levels of MYOC can cause IOP elevation and glaucoma, we generated bacterial artificial chromosome transgenic mice that overexpress Myoc at a level similar to that induced by corticosteroid use. These mice do not develop elevated IOP or glaucoma. Our present findings, along with the absence of glaucoma in mice completely lacking MYOC, show that changing the level of MYOC is not pathogenic (from absent to approximately 15 times normal). These findings suggest that noncoding sequence variants are unlikely to influence glaucoma and that disease pathogenesis in primary open-angle glaucoma patients is dependent upon the expression of abnormal mutant proteins. This work does not support a causative role for increased MYOC levels or the MYOC gene in steroid-induced glaucoma.
Figures


Similar articles
-
Mutant myocilin nonsecretion in vivo is not sufficient to cause glaucoma.Mol Cell Biol. 2006 Nov;26(22):8427-36. doi: 10.1128/MCB.01127-06. Epub 2006 Sep 5. Mol Cell Biol. 2006. PMID: 16954374 Free PMC article.
-
Histochemical Analysis of Glaucoma Caused by a Myocilin Mutation in a Human Donor Eye.Ophthalmol Glaucoma. 2018 Sep-Oct;1(2):132-138. doi: 10.1016/j.ogla.2018.08.004. Epub 2018 Aug 17. Ophthalmol Glaucoma. 2018. PMID: 30906929 Free PMC article.
-
Mutant human myocilin induces strain specific differences in ocular hypertension and optic nerve damage in mice.Exp Eye Res. 2012 Jul;100:65-72. doi: 10.1016/j.exer.2012.04.016. Epub 2012 May 3. Exp Eye Res. 2012. PMID: 22575566 Free PMC article.
-
Heredity in primary open-angle glaucoma.Curr Opin Ophthalmol. 2000 Apr;11(2):101-6. doi: 10.1097/00055735-200004000-00006. Curr Opin Ophthalmol. 2000. PMID: 10848214 Review.
-
Mendelian genes in primary open angle glaucoma.Exp Eye Res. 2019 Sep;186:107702. doi: 10.1016/j.exer.2019.107702. Epub 2019 Jun 22. Exp Eye Res. 2019. PMID: 31238079 Free PMC article. Review.
Cited by
-
Establishment of inducible wild type and mutant myocilin-GFP-expressing RGC5 cell lines.PLoS One. 2012;7(10):e47307. doi: 10.1371/journal.pone.0047307. Epub 2012 Oct 17. PLoS One. 2012. PMID: 23082156 Free PMC article.
-
Myocilin interacts with syntrophins and is member of dystrophin-associated protein complex.J Biol Chem. 2012 Apr 13;287(16):13216-27. doi: 10.1074/jbc.M111.224063. Epub 2012 Feb 25. J Biol Chem. 2012. PMID: 22371502 Free PMC article.
-
Steroid-induced ocular hypertension/glaucoma: Focus on pharmacogenomics and implications for precision medicine.Prog Retin Eye Res. 2017 Jan;56:58-83. doi: 10.1016/j.preteyeres.2016.09.003. Epub 2016 Sep 22. Prog Retin Eye Res. 2017. PMID: 27666015 Free PMC article. Review.
-
Dexamethasone-Induced Ocular Hypertension in Mice: Effects of Myocilin and Route of Administration.Am J Pathol. 2017 Apr;187(4):713-723. doi: 10.1016/j.ajpath.2016.12.003. Epub 2017 Feb 4. Am J Pathol. 2017. PMID: 28167045 Free PMC article.
-
Myocilin Regulates Metalloprotease 2 Activity Through Interaction With TIMP3.Invest Ophthalmol Vis Sci. 2017 Oct 1;58(12):5308-5318. doi: 10.1167/iovs.16-20336. Invest Ophthalmol Vis Sci. 2017. PMID: 29049729 Free PMC article.
References
-
- Ahmed, F., M. Torrado, E. Johnson, J. Morrison, and S. I. Tomarev. 2001. Changes in mRNA levels of the Myoc/Tigr gene in the rat eye after experimental elevation of intraocular pressure or optic nerve transection. Investig Ophthalmol. Vis. Sci. 42:3165-3172. - PubMed
-
- Alward, W. L., Y. H. Kwon, C. L. Khanna, A. T. Johnson, S. S. Hayreh, M. B. Zimmerman, J. Narkiewicz, J. L. Andorf, P. A. Moore, J. H. Fingert, V. C. Sheffield, and E. M. Stone. 2002. Variations in the myocilin gene in patients with open-angle glaucoma. Arch. Ophthalmol. 120:1189-1197. - PubMed
-
- Borras, T., L. L. Rowlette, E. R. Tamm, J. Gottanka, and D. L. Epstein. 2002. Effects of elevated intraocular pressure on outflow facility and TIGR/MYOC expression in perfused human anterior segments. Investig. Ophthalmol. Vis. Sci. 43:33-40. - PubMed
-
- Caballero, M., and T. Borras. 2001. Inefficient processing of an olfactomedin-deficient myocilin mutant: potential physiological relevance to glaucoma. Biochem. Biophys. Res. Commun. 282:662-670. - PubMed
-
- Caballero, M., L. L. Rowlette, and T. Borras. 2000. Altered secretion of a TIGR/MYOC mutant lacking the olfactomedin domain. Biochim. Biophys. Acta 1502:447-460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
