Transgenic analysis reveals that thyroid hormone receptor is sufficient to mediate the thyroid hormone signal in frog metamorphosis
- PMID: 15456876
- PMCID: PMC517898
- DOI: 10.1128/MCB.24.20.9026-9037.2004
Transgenic analysis reveals that thyroid hormone receptor is sufficient to mediate the thyroid hormone signal in frog metamorphosis
Abstract
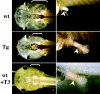
Thyroid hormone (T3) has long been known to be important for vertebrate development and adult organ function. Whereas thyroid hormone receptor (TR) knockout and transgenic studies of mice have implicated TR involvement in mammalian development, the underlying molecular bases for the resulting phenotypes remain to be determined in vivo, especially considering that T3 is known to have both genomic, i.e., through TRs, and nongenomic effects on cells. Amphibian metamorphosis is an excellent model for studying the role of TR in vertebrate development because of its total dependence on T3. Here we investigated the role of TR in metamorphosis by developing a dominant positive mutant thyroid hormone receptor (dpTR). In the frog oocyte transcription system, dpTR bound a T3-responsive promoter and activated the promoter independently of T3. Transgenic expression of dpTR under the control of a heat shock-inducible promoter in premetamorphic tadpoles led to precocious metamorphic transformations. Molecular analyses showed that dpTR induced metamorphosis by specifically binding to known T3 target genes, leading to increased local histone acetylation and gene activation, similar to T3-bound TR during natural metamorphosis. Our experiments indicated that the metamorphic role of T3 is through genomic action of the hormone, at least on the developmental parameters tested. They further provide the first example where TR is shown to mediate directly and sufficiently these developmental effects of T3 in individual organs by regulating target gene expression in these organs.
Figures








Similar articles
-
A dominant-negative thyroid hormone receptor blocks amphibian metamorphosis by retaining corepressors at target genes.Mol Cell Biol. 2003 Oct;23(19):6750-8. doi: 10.1128/MCB.23.19.6750-6758.2003. Mol Cell Biol. 2003. PMID: 12972595 Free PMC article.
-
Distinct expression profiles of transcriptional coactivators for thyroid hormone receptors during Xenopus laevis metamorphosis.Cell Res. 2003 Dec;13(6):459-64. doi: 10.1038/sj.cr.7290188. Cell Res. 2003. PMID: 14728802
-
Nuclear receptor corepressor recruitment by unliganded thyroid hormone receptor in gene repression during Xenopus laevis development.Mol Cell Biol. 2002 Dec;22(24):8527-38. doi: 10.1128/MCB.22.24.8527-8538.2002. Mol Cell Biol. 2002. PMID: 12446772 Free PMC article.
-
Corepressor requirement and thyroid hormone receptor function during Xenopus development.Vitam Horm. 2004;68:209-30. doi: 10.1016/S0083-6729(04)68007-1. Vitam Horm. 2004. PMID: 15193456 Review.
-
Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog.Gen Comp Endocrinol. 2006 Jan 1;145(1):1-19. doi: 10.1016/j.ygcen.2005.07.009. Epub 2005 Nov 2. Gen Comp Endocrinol. 2006. PMID: 16266705 Review.
Cited by
-
An essential and evolutionarily conserved role of protein arginine methyltransferase 1 for adult intestinal stem cells during postembryonic development.Stem Cells. 2010 Nov;28(11):2073-83. doi: 10.1002/stem.529. Stem Cells. 2010. PMID: 20872846 Free PMC article.
-
Unliganded thyroid hormone receptor regulates metamorphic timing via the recruitment of histone deacetylase complexes.Curr Top Dev Biol. 2013;105:275-97. doi: 10.1016/B978-0-12-396968-2.00010-5. Curr Top Dev Biol. 2013. PMID: 23962846 Free PMC article. Review.
-
Liganded thyroid hormone receptor induces nucleosome removal and histone modifications to activate transcription during larval intestinal cell death and adult stem cell development.Endocrinology. 2012 Feb;153(2):961-72. doi: 10.1210/en.2011-1736. Epub 2011 Dec 6. Endocrinology. 2012. PMID: 22147009 Free PMC article.
-
Negative regulation of TSHalpha target gene by thyroid hormone involves histone acetylation and corepressor complex dissociation.Mol Endocrinol. 2009 May;23(5):600-9. doi: 10.1210/me.2008-0389. Epub 2009 Feb 5. Mol Endocrinol. 2009. PMID: 19196836 Free PMC article.
-
Unliganded thyroid hormone receptor α controls developmental timing in Xenopus tropicalis.Endocrinology. 2015 Feb;156(2):721-34. doi: 10.1210/en.2014-1439. Epub 2014 Dec 2. Endocrinology. 2015. PMID: 25456066 Free PMC article.
References
-
- Amano, T., K. Leu, K. Yoshizato, and Y.-B. Shi. 2002. Thyroid hormone regulation of a transcriptional coactivator in Xenopus laevis: implication for a role in postembryonic tissue remodeling. Dev. Dyn. 223:526-535. - PubMed
-
- Atkinson, B. G. 1994. Metamorphosis: model systems for studying gene expression in postembryonic development. Dev. Genet. 15:313-319.
-
- Chen, J. D., and H. Li. 1998. Coactivation and corepression in transcriptional regulation by steroid/nuclear hormone receptors. Crit. Rev. Eukaryot. Gene Expr. 8:169-190. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
