Distinct in vivo dynamics of vertebrate SUMO paralogues
- PMID: 15456902
- PMCID: PMC532004
- DOI: 10.1091/mbc.e04-07-0589
Distinct in vivo dynamics of vertebrate SUMO paralogues
Abstract
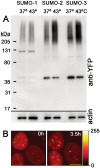
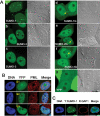
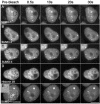
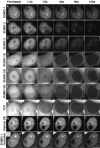
There are three mammalian SUMO paralogues: SUMO-1 is approximately 45% identical to SUMO-2 and SUMO-3, which are 96% identical to each other. It is currently unclear whether SUMO-1, -2, and -3 function in ways that are unique, redundant, or antagonistic. To address this question, we examined the dynamics of individual SUMO paralogues by using cell lines that stably express each of the mammalian SUMO proteins fused to the yellow fluorescent protein (YFP). Whereas SUMO-2 and -3 showed very similar distributions throughout the nucleoplasm, SUMO-1 was uniquely distributed to the nuclear envelope and to the nucleolus. Photobleaching experiments revealed that SUMO-1 dynamics was much slower than SUMO-2 and -3 dynamics. Additionally, the mobility of SUMO paralogues differed between subnuclear structures. Finally, the timing and distributions were dissimilar between paralogues as cells exited from mitosis. SUMO-1 was recruited to nuclear membrane as nuclear envelopes reformed in late anaphase, and accumulated rapidly into the nucleus. SUMO-2 and SUMO-3 localized to chromosome earlier and accumulated gradually during telophase. Together, these findings demonstrate that mammalian SUMO-1 shows patterns of utilization that are clearly discrete from the patterns of SUMO-2 and -3 throughout the cell cycle, arguing that it is functionally distinct and specifically regulated in vivo.
Figures



References
-
- Bachant, J., Alcasabas, A., Blat, Y., Kleckner, N., and Elledge, S.J. (2002). The SUMO-1 isopeptidase Smt4 is linked to centromeric cohesion through SUMO-1 modification of DNA topoisomerase II. Mol. Cell 9, 1169–1182. - PubMed
-
- Dieckhoff, P., Bolte, M., Sancak, Y., Braus, G.H., and Irniger, S. (2004). Smt3/SUMO and Ubc9 are required for efficient APC/C-mediated proteolysis in budding yeast. Mol. Microbiol. 51, 1375–1387. - PubMed
-
- Joseph, J., Liu, S. T., Jablonski, S. A., Yen, T. J., Dosso, M. (2004). The RanGAP1-RanBP2 complex is essential for microtubule-kinetochore interactions in vivo. Curr. Biol. 14, 611–617. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

