Construction, characterization, and complementation of a conditional-lethal DNA topoisomerase IIalpha mutant human cell line
- PMID: 15456904
- PMCID: PMC532048
- DOI: 10.1091/mbc.e04-08-0732
Construction, characterization, and complementation of a conditional-lethal DNA topoisomerase IIalpha mutant human cell line
Abstract
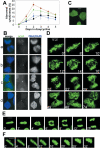
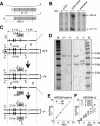
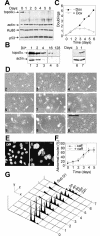
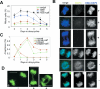
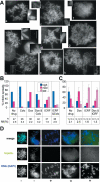
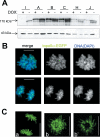
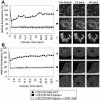
DNA Topoisomerase IIalpha (topoIIalpha) is a DNA decatenating enzyme, abundant constituent of mammalian mitotic chromosomes, and target of numerous antitumor drugs, but its exact role in chromosome structure and dynamics is unclear. In a powerful new approach to this important problem, with significant advantages over the use of topoII inhibitors or RNA interference, we have generated and characterized a human cell line (HTETOP) in which >99.5% topoIIalpha expression can be silenced in all cells by the addition of tetracycline. TopoIIalpha-depleted HTETOP cells enter mitosis and undergo chromosome condensation, albeit with delayed kinetics, but normal anaphases and cytokineses are completely prevented, and all cells die, some becoming polyploid in the process. Cells can be rescued by expression of topoIIalpha fused to green fluorescent protein (GFP), even when certain phosphorylation sites have been mutated, but not when the catalytic residue Y805 is mutated. Thus, in addition to validating GFP-tagged topoIIalpha as an indicator for endogenous topoIIalpha dynamics, our analyses provide new evidence that topoIIalpha plays a largely redundant role in chromosome condensation, but an essential catalytic role in chromosome segregation that cannot be complemented by topoIIbeta and does not require phosphorylation at serine residues 1106, 1247, 1354, or 1393.
Figures
References
-
- Adachi, N., Suzuki, H., Iiizumi, S., and Koyama, H. (2003). Hypersensitivity of nonhomologous DNA end-joining mutants to VP-16 and ICRF- 193, implications for the repair of topoisomerase II-mediated DNA damage. J. Biol. Chem. 278, 35897-35902. - PubMed
-
- Adachi, Y., Luke, M., and Laemmli, U.K. (1991). Chromosome assembly in vitro: topoisomerase II is required for condensation. Cell 64, 137-148. - PubMed
-
- Akimitsu, N., Adachi, N., Hirai, H., Hossain, M.S., Hamamoto, H., Kobayashi, M., Aratani, Y., Koyama, H., and Sekimizu, K. (2003). Enforced cytokinesis without complete nuclear division in embryonic cells depleting the activity of DNA topoisomerase IIalpha. Genes Cells 8, 393-402. - PubMed
-
- Andersen, C.L., Wandall, A., Kjeldsen, E., Mielke, C., and Koch, J. (2002). Active, but not inactive, human centromeres display topoisomerase II activity in vivo. Chromosome Res. 10, 305-312. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

