De novo design of conformationally flexible transmembrane peptides driving membrane fusion
- PMID: 15456911
- PMCID: PMC522031
- DOI: 10.1073/pnas.0405175101
De novo design of conformationally flexible transmembrane peptides driving membrane fusion
Abstract
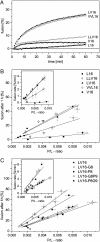
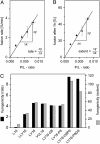
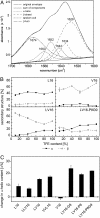
Fusion of biological membranes is mediated by distinct integral membrane proteins, e.g., soluble N-ethylmaleimide-sensitive factor attachment protein receptors and viral fusion proteins. Previous work has indicated that the transmembrane segments (TMSs) of such integral membrane proteins play an important role in fusion. Furthermore, peptide mimics of the transmembrane part can drive the fusion of liposomes, and evidence had been obtained that fusogenicity depends on their conformational flexibility. To test this hypothesis, we present a series of unnatural TMSs that were designed de novo based on the structural properties of hydrophobic residues. We find that the fusogenicity of these peptides depends on the ratio of alpha-helix-promoting Leu and beta-sheet-promoting Val residues and is enhanced by helix-destabilizing Pro and Gly residues within their hydrophobic cores. The ability of these peptides to refold from an alpha-helical state to a beta-sheet conformation and backwards was determined under different conditions. Membrane fusogenic peptides with mixed Leu/Val sequences tend to switch more readily between different conformations than a nonfusogenic peptide with an oligo-Leu core. We propose that structural flexibility of these TMSs is a prerequisite of fusogenicity.
Figures



References
-
- Jahn, R., Lang, T. & Südhof, T. C. (2003) Cell 112, 519–533. - PubMed
-
- Tamm, L. K., Crane, J. & Kiessling, V. (2003) Curr. Opin. Struct. Biol. 13, 453–466. - PubMed
-
- Weber, T., Zemelman, B. V., McNew, J. A., Westermann, B., Gmachl, M., Parlati, F., Söllner, T. H. & Rothman, J. E. (1998) Cell 92, 759–772. - PubMed
-
- Tucker, W. C., Weber, T. & Chapman, E. R. (2004) Science 304, 435–438. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

