Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor
- PMID: 15456912
- PMCID: PMC522054
- DOI: 10.1073/pnas.0406491101
Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor
Abstract
The cytokine erythropoietin (Epo) is tissue-protective in preclinical models of ischemic, traumatic, toxic, and inflammatory injuries. We have recently characterized Epo derivatives that do not bind to the Epo receptor (EpoR) yet are tissue-protective. For example, carbamylated Epo (CEpo) does not stimulate erythropoiesis, yet it prevents tissue injury in a wide variety of in vivo and in vitro models. These observations suggest that another receptor is responsible for the tissue-protective actions of Epo. Notably, prior investigation suggests that EpoR physically interacts with the common beta receptor (betacR), the signal-transducing subunit shared by the granulocyte-macrophage colony stimulating factor, and the IL-3 and IL-5 receptors. However, because betacR knockout mice exhibit normal erythrocyte maturation, betacR is not required for erythropoiesis. We hypothesized that betacR in combination with the EpoR expressed by nonhematopoietic cells constitutes a tissue-protective receptor. In support of this hypothesis, membrane proteins prepared from rat brain, heart, liver, or kidney were greatly enriched in EpoR after passage over either Epo or CEpo columns but covalently bound in a complex with betacR. Further, antibodies against EpoR coimmunoprecipitated betacR from membranes prepared from neuronal-like P-19 cells that respond to Epo-induced tissue protection. Immunocytochemical studies of spinal cord neurons and cardiomyocytes protected by Epo demonstrated cellular colocalization of Epo betacR and EpoR. Finally, as predicted by the hypothesis, neither Epo nor CEpo was active in cardiomyocyte or spinal cord injury models performed in the betacR knockout mouse. These data support the concept that EpoR and betacR comprise a tissue-protective heteroreceptor.
Figures
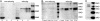


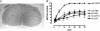
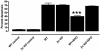
References
-
- Grasso, G., Sfacteria, A., Cerami, A. & Brines, M. (2004) Neuroscientist 10, 93-98. - PubMed
-
- Ghezzi, P. & Brines, M. (2004) Cell Death Differ. 11, Suppl. 1, S37-S44. - PubMed
-
- Livnah, O., Stura, E. A., Middleton, S. A., Johnson, D. L., Jolliffe, L. K. & Wilson, I. A. (1999) Science 283, 987-990. - PubMed
-
- Masuda, S., Nagao, M., Takahata, K., Konishi, Y., Gallyas, F., Jr., Tabira, T. & Sasaki, R. (1993) J. Biol. Chem. 268, 11208-11216. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

