GTP/GDP exchange by Sec12p enables COPII vesicle bud formation on synthetic liposomes
- PMID: 15457212
- PMCID: PMC524392
- DOI: 10.1038/sj.emboj.7600428
GTP/GDP exchange by Sec12p enables COPII vesicle bud formation on synthetic liposomes
Abstract
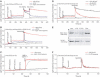
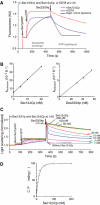
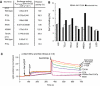
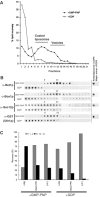
The generation of COPII vesicles from synthetic liposome membranes requires the minimum coat components Sar1p, Sec23/24p, Sec13/31p, and a nonhydrolyzable GTP analog such as GMP-PNP. However, in the presence of GTP and the full complement of coat subunits, nucleotide hydrolysis by Sar1p renders the coat insufficiently stable to sustain vesicle budding. In order to recapitulate a more authentic, GTP-dependent budding event, we introduced the Sar1p nucleotide exchange catalyst, Sec12p, and evaluated the dynamics of coat assembly and disassembly by light scattering and tryptophan fluorescence measurements. The catalytic, cytoplasmic domain of Sec12p (Sec12DeltaCp) activated Sar1p with a turnover 10-fold higher than the GAP activity of Sec23p stimulated by the full coat. COPII assembly was stabilized on liposomes incubated with Sec12DeltaCp and GTP. Numerous COPII budding profiles were visualized on membranes, whereas a parallel reaction conducted in the absence of Sec12DeltaCp produced no such profiles. We suggest that Sec12p participates actively in the growth of COPII vesicles by charging new Sar1p-GTP molecules that insert at the boundary between a bud and the surrounding endoplasmic reticulum membrane.
Figures


References
-
- Antonny B, Madden D, Hamamoto S, Orci L, Schekman R (2001) Dynamics of the COPII coat with GTP and stable analogues. Nat Cell Biol 3: 531–537 - PubMed
-
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R (1994) COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77: 895–907 - PubMed
-
- Barlowe C, Schekman R (1993) SEC12 encodes a guanine nucleotide exchange factor essential for transport vesicle budding from ER. Nature 365: 347–349 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

