Variable LH2 stoichiometry and core clustering in native membranes of Rhodospirillum photometricum
- PMID: 15457213
- PMCID: PMC524393
- DOI: 10.1038/sj.emboj.7600429
Variable LH2 stoichiometry and core clustering in native membranes of Rhodospirillum photometricum
Abstract
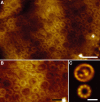
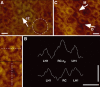
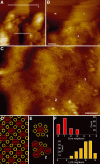
The individual components of the photosynthetic unit (PSU), the light-harvesting complexes (LH2 and LH1) and the reaction center (RC), are structurally and functionally known in great detail. An important current challenge is the study of their assembly within native membranes. Here, we present AFM topographs at 12 A resolution of native membranes containing all constituents of the PSU from Rhodospirillum photometricum. Besides the major technical advance represented by the acquisition of such highly resolved data of a complex membrane, the images give new insights into the organization of this energy generating apparatus in Rsp. photometricum: (i) there is a variable stoichiometry of LH2, (ii) the RC is completely encircled by a closed LH1 assembly, (iii) the LH1 assembly around the RC forms an ellipse, (iv) the PSU proteins cluster together segregating out of protein free lipid bilayers, (v) core complexes cluster although enough LH2 are present to prevent core-core contacts, and (vi) there is no cytochrome bc1 complex visible in close proximity to the RCs. The functional significance of all these findings is discussed.
Figures

References
-
- Bahatyrova S, Frese RN, Van Der Werf KO, Otto C, Hunter CN, Olsen JD (2004) Flexibility and size heterogeneity of the LH1 light harvesting complex revealed by atomic force microscopy: functional significance for bacterial photosynthesis. J Biol Chem 279: 21327–21333 - PubMed
-
- Creighton TE (1993) Proteins. WH Freeman & Company: New York, NY
-
- Deisenhofer J, Epp O, Miki K, Huber R, Michel H (1984) X-ray structure analysis of a membrane protein complex. J Mol Biol 180: 385. - PubMed
-
- Deisenhofer J, Epp O, Miki K, Huber R, Michel H (1985) Structure of the protein subunits in the photosynthetic reaction centre of Rhodopseudomonas viridis at 3 Å resolution. Nature 318: 618–624 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

