Reaction discovery enabled by DNA-templated synthesis and in vitro selection
- PMID: 15457254
- PMCID: PMC2814052
- DOI: 10.1038/nature02920
Reaction discovery enabled by DNA-templated synthesis and in vitro selection
Abstract
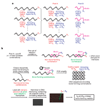
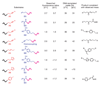
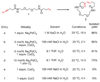
Current approaches to reaction discovery focus on one particular transformation. Typically, researchers choose substrates based on their predicted ability to serve as precursors for the target structure, then evaluate reaction conditions for their ability to effect product formation. This approach is ideal for addressing specific reactivity problems, but its focused nature might leave many areas of chemical reactivity unexplored. Here we report a reaction discovery approach that uses DNA-templated organic synthesis and in vitro selection to simultaneously evaluate many combinations of different substrates for bond-forming reactions in a single solution. Watson-Crick base pairing controls the effective molarities of substrates tethered to DNA strands; bond-forming substrate combinations are then revealed using in vitro selection for bond formation, PCR amplification and DNA microarray analysis. Using this approach, we discovered an efficient and mild carbon-carbon bond-forming reaction that generates an enone from an alkyne and alkene using an inorganic palladium catalyst. Although this approach is restricted to conditions and catalysts that are at least partially compatible with DNA, we expect that its versatility and efficiency will enable the discovery of additional reactions between a wide range of substrates.
Figures

References
-
- Stambuli JP, Hartwig JF. Recent advances in the discovery of organometallic catalysts using high-throughput screening assays. Curr. Opin. Chem. Biol. 2003;7:420–426. - PubMed
-
- Reetz MT. Combinatorial and evolution-based methods in the creation of enantioselective catalysts. Angew. Chem. Int. Edn Engl. 2001;40:284–310. - PubMed
-
- Stambuli JP, Stauffer SR, Shaughnessy KH, Hartwig JF. Screening of homogeneous catalysts by fluorescence resonance energy transfer. Identification of catalysts for room-temperature Heck reactions. J. Am. Chem. Soc. 2001;123:2677–2678. - PubMed
-
- Taylor SJ, Morken JP. Thermographic selection of effective catalysts from an encoded polymer-bound library. Science. 1998;280:267–270. - PubMed
-
- Lober O, Kawatsura M, Hartwig JF. Palladium-catalyzed hydroamination of 1,3-dienes: a colorimetric assay and enantioselective additions. J. Am. Chem. Soc. 2001;123:4366–4367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

