Functional studies of human intestinal alkaline sphingomyelinase by deglycosylation and mutagenesis
- PMID: 15458386
- PMCID: PMC1134777
- DOI: 10.1042/BJ20041455
Functional studies of human intestinal alkaline sphingomyelinase by deglycosylation and mutagenesis
Abstract
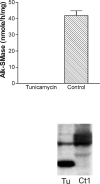
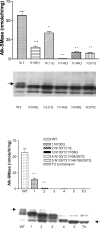
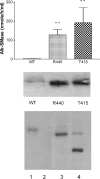
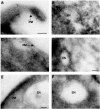
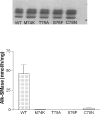
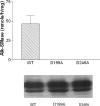
Intestinal alk-SMase (alkaline sphingomyelinase) is an ectoenzyme related to the NPP (nucleotide phosphodiesterase) family. It has five potential N-glycosylation sites and predicated transmembrane domains at both the N- and C-termini. The amino acid residues forming the two metal-binding sites in NPP are conserved, and those of the active core are modified. We examined the functional changes of the enzyme induced by deglycosylation and mutagenesis. Treating alk-SMase cDNA-transfected COS-7 cells with tunicamycin rendered the expressed enzyme completely inactive. Mutations of the five potential N-glycosylation sites individually and in combination showed that these sites were all glycosylated and deficient glycosylation decreased the enzyme activity. Immunogold labelling showed that the wild-type enzyme was mainly located in the plasma membrane, whereas the C-terminal domain-truncated enzyme was released into the medium. Deglycosylation blocked the release of the enzyme that accumulated in endosome-like structures. The enzyme activity was also decreased by mutations of the residues forming the putative metal-binding sites and the active core. Substitution of the active core sequence with that of NPP or mutation of T75 in the core abolished the enzyme activity against sphingomyelin but failed to render the enzyme NPP active. Our results indicate that alk-SMase activity is severely affected by defective N-glycosylation and structural alterations of the putative metal-binding sites and the predicted active core.
Figures

References
-
- Nilsson Å. The presence of sphingomyelin- and ceramide-cleaving enzymes in the small intestinal tract. Biochim. Biophys. Acta. 1969;176:339–347. - PubMed
-
- Hannun Y. A., Linardic C. M. Sphingolipid breakdown products: anti-proliferative and tumor-suppressor lipids. Biochim. Biophys. Acta. 1993;1154:223–236. - PubMed
-
- Merrill A. H., Jr, Schmelz E. M., Wang E., Schroeder J. J., Dillehay D. L., Riley R. T. Role of dietary sphingolipids and inhibitors of sphingolipid metabolism in cancer and other diseases. J. Nutr. 1995;125:1677S–1682S. - PubMed
-
- Duan R. Potential link between sphingomyelin metabolism and colonic tumorigenesis. In: Scheppach W., Scheurlen M., editors. Exogenous Factors in Colonic Carcinogenesis. Lancaster, U.K.: Kluwer Academic; 2003. pp. 142–153.
-
- Dillehay D. L., Webb S. K., Schmelz E.-M., Merrill A. H. Dietary sphingomyelin inhibits 1,2-dimethylhydrazine-induced colon cancer in CF1 mice. J. Nutr. 1994;124:615–620. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

