NMDA receptors induce somatodendritic secretion in hypothalamic neurones of lactating female rats
- PMID: 15459239
- PMCID: PMC1665332
- DOI: 10.1113/jphysiol.2004.069005
NMDA receptors induce somatodendritic secretion in hypothalamic neurones of lactating female rats
Abstract
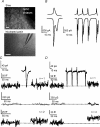
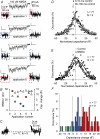
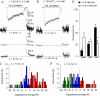
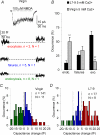
Many neurones in the mammalian brain are known to release the content of their vesicles from somatodendritic locations. These vesicles usually contain retrograde messengers that modulate network properties. The back-propagating action potential is thought to be the principal physiological stimulus that evokes somatodendritic release. In contrast, here we show that calcium influx through NMDA receptor (NMDAR) channels, in the absence of postsynaptic cell firing, is also able to induce vesicle fusion from non-synaptic sites in nucleated outside-out patches of dorsomedial supraoptic nucleus (SON) neurones of adult female rats, in particular during their reproductive stages. The physiological significance of this mechanism was characterized in intact brain slices, where NMDAR-mediated release of oxytocin was shown to retrogradely inhibit presynaptic GABA release, in the absence of postsynaptic cell firing. This implies that glutamatergic synaptic input in itself is sufficient to elicit the release of oxytocin, which in turn acts as a retrograde messenger leading to the depression of nearby GABA synapses. In addition, we found that during lactation, when oxytocin demand is high, NMDA-induced oxytocin release is up-regulated compared to that in non-reproductive rats. Thus, in the hypothalamus, local signalling back and forth between pre- and postsynaptic compartments and between different synapses may occur independently of the firing activity of the postsynaptic neurone.
Figures


Similar articles
-
NMDA receptors trigger neurosecretion of 5-HT within dorsal raphe nucleus of the rat in the absence of action potential firing.J Physiol. 2006 Dec 15;577(Pt 3):891-905. doi: 10.1113/jphysiol.2006.115311. Epub 2006 Oct 19. J Physiol. 2006. PMID: 17053037 Free PMC article.
-
NMDA receptors potentiate activity-dependent dendritic release of neuropeptides from hypothalamic neurons.J Physiol. 2019 Mar;597(6):1735-1756. doi: 10.1113/JP277167. Epub 2019 Jan 30. J Physiol. 2019. PMID: 30629746 Free PMC article.
-
Differential GABAA receptor clustering determines GABA synapse plasticity in rat oxytocin neurons around parturition and the onset of lactation.Mol Cell Neurosci. 2005 Jan;28(1):128-40. doi: 10.1016/j.mcn.2004.09.002. Mol Cell Neurosci. 2005. PMID: 15607948
-
Glial cells: indispensable partners of hypothalamic magnocellular neurones.J Neuroendocrinol. 2009 Jul;21(7):665-72. doi: 10.1111/j.1365-2826.2009.01884.x. Epub 2009 May 7. J Neuroendocrinol. 2009. PMID: 19453824 Review.
-
Dendritic spikes and activity-dependent synaptic plasticity.Cell Tissue Res. 2006 Nov;326(2):369-77. doi: 10.1007/s00441-006-0263-8. Epub 2006 Jul 1. Cell Tissue Res. 2006. PMID: 16816965 Review.
Cited by
-
NMDA receptors trigger neurosecretion of 5-HT within dorsal raphe nucleus of the rat in the absence of action potential firing.J Physiol. 2006 Dec 15;577(Pt 3):891-905. doi: 10.1113/jphysiol.2006.115311. Epub 2006 Oct 19. J Physiol. 2006. PMID: 17053037 Free PMC article.
-
The Thermodynamically Expensive Contribution of Three Calcium Sources to Somatic Release of Serotonin.Int J Mol Sci. 2022 Jan 28;23(3):1495. doi: 10.3390/ijms23031495. Int J Mol Sci. 2022. PMID: 35163419 Free PMC article. Review.
-
Short-term potentiation of mEPSCs requires N-, P/Q- and L-type Ca2+ channels and mitochondria in the supraoptic nucleus.J Physiol. 2008 Jul 1;586(13):3147-61. doi: 10.1113/jphysiol.2007.148957. Epub 2008 May 8. J Physiol. 2008. PMID: 18467369 Free PMC article.
-
Multiple signalling modalities mediated by dendritic exocytosis of oxytocin and vasopressin.Philos Trans R Soc Lond B Biol Sci. 2015 Jul 5;370(1672):20140182. doi: 10.1098/rstb.2014.0182. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26009761 Free PMC article. Review.
-
Activity-dependent synaptic plasticity in the supraoptic nucleus of the rat hypothalamus.J Physiol. 2006 Jun 15;573(Pt 3):711-21. doi: 10.1113/jphysiol.2006.109447. Epub 2006 Apr 13. J Physiol. 2006. PMID: 16613872 Free PMC article.
References
-
- Al-Ghoul WM, Meeker RB, Greenwood RS. Differential expression of five N-methyl-D-aspartate receptor subunit mRNAs in vasopressin and oxytocin neuroendocrine cells. Brain Res Mol Brain Res. 1997;44:262–272. 10.1016/S0169-328X(96)00205-7. - DOI - PubMed
-
- Artalejo CR, Elhamdani A, Palfrey HC. Sustained stimulation shifts the mechanism of endocytosis from dynamin-1-dependent rapid endocytosis to clathrin- and dynamin-2-mediated slow endocytosis in chromaffin cells. Proc Natl Acad Sci U S A. 2002;99:6358–6363. 10.1073/pnas.082658499. - DOI - PMC - PubMed
-
- Brussaard AB, Herbison AE. Long-term plasticity of postsynaptic GABAA-receptor function in the adult brain: insights from the oxytocin neurone. Trends Neurosci. 2000;23:190–195. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

