The effect of simulated ischaemia on spontaneous GABA release in area CA1 of the juvenile rat hippocampus
- PMID: 15459240
- PMCID: PMC1665352
- DOI: 10.1113/jphysiol.2004.070490
The effect of simulated ischaemia on spontaneous GABA release in area CA1 of the juvenile rat hippocampus
Abstract
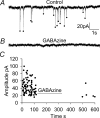
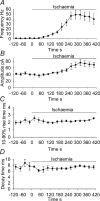
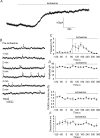
An early consequence of brain energy deprivation is an increase in the frequency of spontaneous inhibitory and excitatory postsynaptic currents (sIPSCs and sEPSCs), which may disrupt neural information processing. This increase in spontaneous transmitter release has been reported to occur in calcium-free solution and has been attributed either to calcium release from internal stores or to a direct effect of hypoxia on the transmitter release mechanism. Here we investigate the mechanism of the increase in sIPSC frequency that occurs in area CA1 of rat hippocampus during simulated ischaemia, by making patch-clamp recordings from CA1 pyramidal neurones. When recording in whole-cell mode, exposure to ischaemic solution increased the sIPSC frequency 30-fold (to 49 Hz) after 5 min, and doubled the sIPSC amplitude. Ischaemic sIPSCs were action potential independent, vesicular in origin and, contrary to the results of earlier studies which did not buffer extracellular calcium to a low level, dependent on extracellular calcium. The properties of the ischaemic sIPSCs were not affected by depleting intracellular stores of calcium or by blocking the neuronal GABA transporter GAT-1. Recording from neurones using gramicidin-perforated patch-clamping showed a 10-fold smaller, more transient increase in sIPSC frequency during ischaemia, with no change of sIPSC amplitude, suggesting that whole-cell clamp recording increases the ischaemia-induced sIPSC rate and amplitude by controlling the intracellular milieu.
Figures




References
-
- Akaike N. Gramicidin perforated patch recording and intracellular chloride activity in excitable cells. Prog Biophys Mol Biol. 1996;65:251–264. - PubMed
-
- Behrends JC, Maruyama T, Tokutomi N, Akaike N. Ca2+-mediated suppression of the GABA-response through modulation of chloride channel gating in frog sensory neurones. Neurosci Lett. 1988;86:311–316. - PubMed
-
- Borden LA, Murali Dhar TG, Smith KE, Weinshank RL, Branchek TA, Gluchowski C. Tiagabine, SK&F 89976-A, CI-966 and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur J Pharmacol. 1994;269:219–224. - PubMed
-
- Centonze D, Saulle E, Pisani A, Bernardi G, Calabresi P. Adenosine-mediated inhibition of striatal GABAergic synaptic transmission during in vitro ischaemia. Brain. 2001;124:1855–1865. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

