Regulation of gating by negative charges in the cytoplasmic pore in the Kir2.1 channel
- PMID: 15459242
- PMCID: PMC1665335
- DOI: 10.1113/jphysiol.2004.072330
Regulation of gating by negative charges in the cytoplasmic pore in the Kir2.1 channel
Abstract
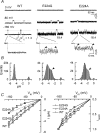
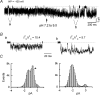
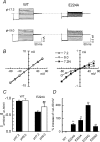
Inward rectifier K(+) channels commonly exhibit long openings (slow gating) punctuated by rapid open-close transitions (fast gating), suggesting that two separate gates may control channel open-closed transitions. Previous studies have suggested possible gate locations at the selectivity filter and at the 'bundle crossing', where the two transmembrane segments (M1 and M2) cross near the cytoplasmic end of the pore. Wild-type Kir2.1 channels exhibit only slow gating, but mutations in the cytoplasmic pore domain at E224 and E299 have been shown to induce fast flickery gating. Since these mutations also affect polyamine affinity, we conjectured that the fast gating mechanism might affect the kinetics of polyamine block/unblock if located more intracellularly than the polyamine blocking site in the pore. Neutralization of either E224 or E299 induced fast gating and slowed both block and unblock rates by the polyamine diamine 10. The slowing of polyamine block/unblock was partly relieved by raising pH from 7.2 to 9.0, which also slowed fast gating kinetics. These findings indicate that the fast flickery gate is located intracellularly with respect to the polyamine pore-plugging site near D172, thereby excluding the selectivity filter, and implicating the bundle crossing or more intracellular site as the gate. As additional proof, fast gating induced at the selectivity filter by disrupting P loop salt bridges in WT-E138D-E138D-WT tandem had no effect on polyamine block and unblock rates. The pH sensitivity of fast gating in E224 and E299 mutants was attributed to the protonation state of H226, since the double mutant E224Q/H226K induced fast gating which was pH insensitive. Moreover, introducing a negative charge in the 224-226 region was sufficient to prevent fast gating, since the double mutant E224Q/H226E rescued wild-type Kir2.1 slow gating. These observations implicate E224 and E299 as allosteric modulators of a fast gate, located at the bundle crossing or below in Kir2.1 channels. By suppressing fast gating, these negative charges facilitate polyamine block and unblock, which may be their physiologically important role.
Figures






Similar articles
-
Electrostatics in the cytoplasmic pore produce intrinsic inward rectification in kir2.1 channels.J Gen Physiol. 2005 Dec;126(6):551-62. doi: 10.1085/jgp.200509367. J Gen Physiol. 2005. PMID: 16316974 Free PMC article.
-
Inward rectification by polyamines in mouse Kir2.1 channels: synergy between blocking components.J Physiol. 2003 Jul 1;550(Pt 1):67-82. doi: 10.1113/jphysiol.2003.043117. Epub 2003 May 9. J Physiol. 2003. PMID: 12740427 Free PMC article.
-
Mechanism of rectification in inward-rectifier K+ channels.J Gen Physiol. 2003 Apr;121(4):261-75. doi: 10.1085/jgp.200208771. Epub 2003 Mar 17. J Gen Physiol. 2003. PMID: 12642596 Free PMC article.
-
Two aspects of the inward rectification mechanism. Effects of cytoplasmic blockers and extracellular K+ on the inward rectifier K+ channel.Jpn Heart J. 1996 Sep;37(5):631-41. doi: 10.1536/ihj.37.631. Jpn Heart J. 1996. PMID: 8973376 Review.
-
Gating of inward rectifier K(+) channels by proton-mediated interactions of intracellular protein domains.Trends Cardiovasc Med. 2002 Jan;12(1):5-13. doi: 10.1016/s1050-1738(01)00132-3. Trends Cardiovasc Med. 2002. PMID: 11796238 Review.
Cited by
-
Multi-ion versus single-ion conduction mechanisms can yield current rectification in biological ion channels.J Biol Phys. 2014 Mar;40(2):109-19. doi: 10.1007/s10867-013-9338-4. Epub 2014 Jan 26. J Biol Phys. 2014. PMID: 24463792 Free PMC article.
-
Tl+-induced micros gating of current indicates instability of the MaxiK selectivity filter as caused by ion/pore interaction.J Gen Physiol. 2008 Apr;131(4):365-78. doi: 10.1085/jgp.200809956. J Gen Physiol. 2008. PMID: 18378799 Free PMC article.
-
Functional roles of charged amino acid residues on the wall of the cytoplasmic pore of Kir2.1.J Gen Physiol. 2006 Apr;127(4):401-19. doi: 10.1085/jgp.200509434. Epub 2006 Mar 13. J Gen Physiol. 2006. PMID: 16533896 Free PMC article.
-
Channel rectification made simple.Biophys J. 2025 Feb 18;124(4):587-589. doi: 10.1016/j.bpj.2025.01.013. Epub 2025 Jan 24. Biophys J. 2025. PMID: 39863926 No abstract available.
-
Electrostatics in the cytoplasmic pore produce intrinsic inward rectification in kir2.1 channels.J Gen Physiol. 2005 Dec;126(6):551-62. doi: 10.1085/jgp.200509367. J Gen Physiol. 2005. PMID: 16316974 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

