Acute desensitization of GIRK current in rat atrial myocytes is related to K+ current flow
- PMID: 15459243
- PMCID: PMC1665358
- DOI: 10.1113/jphysiol.2004.072462
Acute desensitization of GIRK current in rat atrial myocytes is related to K+ current flow
Abstract
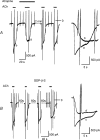
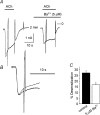
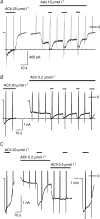
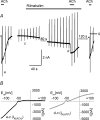
We have investigated the acute desensitization of acetylcholine-activated GIRK current (I(K(ACh))) in cultured adult rat atrial myocytes. Acute desensitization of I(K(ACh)) is observed as a partial relaxation of current with a half-time of < 5 s when muscarinic M2 receptors are stimulated by a high concentration (> 2 micromol l(-1)) of ACh. Under this condition experimental manoeuvres that cause a decrease in the amplitude of I(K(ACh)), such as partial block of M2 receptors by atropine, intracellular loading with GDP-beta-S, or exposure to Ba2+, caused a reduction in desensitization. Acute desensitization was also identified as a decrease in current amplitude and a blunting of the response to saturating [ACh] (20 micromol l(-1)) when the current had been partially activated by a low concentration of ACh or by stimulation of adenosine A1 receptors. A reduction in current analogous to acute desensitization was observed when ATP-dependent K+ current (I(K(ATP))) was activated either by mitochondrial uncoupling using 2,4-dinitrophenole (DNP) or by the channel opener rilmakalim. Adenovirus-driven overexpression of Kir2.1, a subunit of constitutively active inwardly rectifying K+ channels, resulted in a large Ba2+-sensitive background K+ current and a dramatic reduction of ACh-activated current. Adenovirus-driven overexpression of GIRK4 (Kir3.4) subunits resulted in an increased agonist-independent GIRK current paralleled by a reduction in I(K(ACh)) and removal of the desensitizing component. These data indicate that acute desensitization depends on K+ current flow, independent of the K+ channel species, suggesting that it reflects a reduction in electrochemical driving force rather than a bona fide signalling mechanism. This is supported by the observation that desensitization is paralleled by a significant negative shift in reversal potential of I(K(ACh)). Since the ACh-induced hyperpolarization shows comparable desensitization properties as I(K(ACh)), this novel current-dependent desensitization is a physiologically relevant process, shaping the time course of parasympathetic bradycardia.
Figures






Similar articles
-
G protein-independent inhibition of GIRK current by adenosine in rat atrial myocytes overexpressing A1 receptors after adenovirus-mediated gene transfer.J Physiol. 2003 Aug 1;550(Pt 3):707-17. doi: 10.1113/jphysiol.2003.041962. Epub 2003 Jun 18. J Physiol. 2003. PMID: 12815176 Free PMC article.
-
Overexpression of monomeric and multimeric GIRK4 subunits in rat atrial myocytes removes fast desensitization and reduces inward rectification of muscarinic K(+) current (I(K(ACh))). Evidence for functional homomeric GIRK4 channels.J Biol Chem. 2001 Aug 3;276(31):28873-80. doi: 10.1074/jbc.M102328200. Epub 2001 May 30. J Biol Chem. 2001. PMID: 11384974
-
Differential effects of genetically-encoded Gβγ scavengers on receptor-activated and basal Kir3.1/Kir3.4 channel current in rat atrial myocytes.Cell Signal. 2014 Jun;26(6):1182-92. doi: 10.1016/j.cellsig.2014.02.007. Epub 2014 Feb 24. Cell Signal. 2014. PMID: 24576551
-
Voltage dependence of ATP-dependent K+ current in rat cardiac myocytes is affected by IK1 and IK(ACh).J Physiol. 2004 Dec 1;561(Pt 2):459-69. doi: 10.1113/jphysiol.2004.073197. Epub 2004 Sep 30. J Physiol. 2004. PMID: 15459245 Free PMC article.
-
Constitutive activity of the acetylcholine-activated potassium current IK,ACh in cardiomyocytes.Adv Pharmacol. 2014;70:393-409. doi: 10.1016/B978-0-12-417197-8.00013-4. Adv Pharmacol. 2014. PMID: 24931203 Review.
Cited by
-
A genetically encoded tool kit for manipulating and monitoring membrane phosphatidylinositol 4,5-bisphosphate in intact cells.PLoS One. 2011;6(6):e20855. doi: 10.1371/journal.pone.0020855. Epub 2011 Jun 9. PLoS One. 2011. PMID: 21695261 Free PMC article.
-
Short-term desensitization of muscarinic K+ current in the heart.Biophys J. 2013 Sep 17;105(6):1515-25. doi: 10.1016/j.bpj.2013.08.009. Biophys J. 2013. PMID: 24048003 Free PMC article.
-
Autocrine signaling via A(1) adenosine receptors causes downregulation of M(2) receptors in adult rat atrial myocytes in vitro.Pflugers Arch. 2011 Jan;461(1):165-76. doi: 10.1007/s00424-010-0897-y. Pflugers Arch. 2011. PMID: 21061016
-
Structural and Electrical Remodeling of the Sinoatrial Node in Diabetes: New Dimensions and Perspectives.Front Endocrinol (Lausanne). 2022 Jul 7;13:946313. doi: 10.3389/fendo.2022.946313. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35872997 Free PMC article. Review.
-
Galphai3 primes the G protein-activated K+ channels for activation by coexpressed Gbetagamma in intact Xenopus oocytes.J Physiol. 2007 May 15;581(Pt 1):17-32. doi: 10.1113/jphysiol.2006.125864. Epub 2007 Feb 8. J Physiol. 2007. PMID: 17289785 Free PMC article.
References
-
- Bender K, Wellner-Kienitz M-C, Inanobe A, Meyer T, Kurachi Y, Pott L. Overexpression of monomeric and multimeric GIRK4 subunits in rat atrial myocytes removes fast desensitization and reduces inward rectification of muscarinic K+ current (IK(ACh)) J Biol Chem. 2001;276:28873–28880. - PubMed
-
- Bender K, Wellner-Kienitz M-C, Meyer T, Pott L. Activation of muscarinic K+ current by β-adrenergic receptors in cultured atrial myocytes transfected with β1 subunit of heterotrimeric G proteins. FEBS Lett. 1998;439:115–120. - PubMed
-
- Bender K, Wellner-Kienitz M-C, Pott L. Transfection of a phosphatidyl-4-phosphate 5-kinase gene into rat atrial myocytes removes inhibition of GIRK current by endothelin and α-adrenergic agonists. FEBS Lett. 2002;529:356–360. - PubMed
-
- Bösche LI, Bender K, Wellner-Kienitz M-C, Pott L. Transfection with 5-HT1A receptor gene and antisense directed against muscarinic M2 receptors reveal a mutual influence between Gi/o-coupled receptors in rat arial myoctes. J Mol Cell Cardiol. 2003a;35:99–107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

