Melatonin advances the circadian timing of EEG sleep and directly facilitates sleep without altering its duration in extended sleep opportunities in humans
- PMID: 15459246
- PMCID: PMC1665336
- DOI: 10.1113/jphysiol.2004.073742
Melatonin advances the circadian timing of EEG sleep and directly facilitates sleep without altering its duration in extended sleep opportunities in humans
Abstract
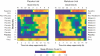
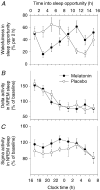
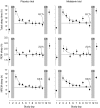
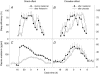
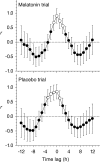
The rhythm of plasma melatonin originating from the pineal gland and driven by the circadian pacemaker located in the suprachiasmatic nucleus is closely associated with the circadian (approximately 24 h) variation in sleep propensity and sleep spindle activity in humans. We investigated the contribution of melatonin to variation in sleep propensity, structure, duration and EEG activity in a protocol in which sleep was scheduled to begin during the biological day, i.e. when endogenous melatonin concentrations are low. The two 14 day trials were conducted in an environmental scheduling facility. Each trial included two circadian phase assessments, baseline sleep and nine 16 h sleep opportunities (16.00-08.00 h) in near darkness. Eight healthy male volunteers (24.4 +/- 4.4 years) without sleep complaints were recruited, and melatonin (1.5 mg) or placebo was administered at the start of the first eight 16 h sleep opportunities. During melatonin treatment, sleep in the first 8 h of the 16 h sleep opportunities was increased by 2 h. Sleep per 16 h was not significantly different and approached asymptotic values of 8.7 h in both conditions. The percentage of rapid eye movement (REM) sleep was not affected by melatonin, but the percentage of stage 2 sleep and sleep spindle activity increased, and the percentage of stage 3 sleep decreased. During the washout night, the melatonin-induced advance in sleep timing persisted, but was smaller than on the preceding treatment night and was consistent with the advance in the endogenous melatonin rhythm. These data demonstrate robust, direct sleep-facilitating and circadian effects of melatonin without concomitant changes in sleep duration, and support the use of melatonin in the treatment of sleep disorders in which the circadian melatonin rhythm is delayed relative to desired sleep time.
Figures

Similar articles
-
Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase of circadian melatonin rhythm in humans.J Physiol. 1997 Dec 15;505 ( Pt 3)(Pt 3):851-8. doi: 10.1111/j.1469-7793.1997.851ba.x. J Physiol. 1997. PMID: 9457658 Free PMC article.
-
Separation of circadian and wake duration-dependent modulation of EEG activation during wakefulness.Neuroscience. 2002;114(4):1047-60. doi: 10.1016/s0306-4522(02)00209-9. Neuroscience. 2002. PMID: 12379258
-
Melatonin and the circadian regulation of sleep initiation, consolidation, structure, and the sleep EEG.J Biol Rhythms. 1997 Dec;12(6):627-35. doi: 10.1177/074873049701200618. J Biol Rhythms. 1997. PMID: 9406038 Review.
-
Robust circadian rhythm in heart rate and its variability: influence of exogenous melatonin and photoperiod.J Sleep Res. 2007 Jun;16(2):148-55. doi: 10.1111/j.1365-2869.2007.00581.x. J Sleep Res. 2007. PMID: 17542944 Clinical Trial.
-
Circadian rhythm sleep disorders: pathophysiology and potential approaches to management.CNS Drugs. 2001;15(4):311-28. doi: 10.2165/00023210-200115040-00005. CNS Drugs. 2001. PMID: 11463135 Review.
Cited by
-
Daytime Exposure to Short Wavelength-Enriched Light Improves Cognitive Performance in Sleep-Restricted College-Aged Adults.Front Neurol. 2021 Feb 22;12:624217. doi: 10.3389/fneur.2021.624217. eCollection 2021. Front Neurol. 2021. PMID: 33692742 Free PMC article.
-
Limited Benefit of Sleep Extension on Cognitive Deficits During Total Sleep Deprivation: Illustration With Two Executive Processes.Front Neurosci. 2019 Jun 19;13:591. doi: 10.3389/fnins.2019.00591. eCollection 2019. Front Neurosci. 2019. PMID: 31275098 Free PMC article.
-
Efficacy of melatonin with behavioural sleep-wake scheduling for delayed sleep-wake phase disorder: A double-blind, randomised clinical trial.PLoS Med. 2018 Jun 18;15(6):e1002587. doi: 10.1371/journal.pmed.1002587. eCollection 2018 Jun. PLoS Med. 2018. PMID: 29912983 Free PMC article. Clinical Trial.
-
Effect of Light and Melatonin and Other Melatonin Receptor Agonists on Human Circadian Physiology.Sleep Med Clin. 2015 Dec;10(4):435-53. doi: 10.1016/j.jsmc.2015.08.001. Epub 2015 Sep 7. Sleep Med Clin. 2015. PMID: 26568121 Free PMC article. Review.
-
The use of exogenous melatonin in delayed sleep phase disorder: a meta-analysis.Sleep. 2010 Dec;33(12):1605-14. doi: 10.1093/sleep/33.12.1605. Sleep. 2010. PMID: 21120122 Free PMC article. Review.
References
-
- Aeschbach D, Sher L, Postolache TT, Matthews JR, Jackson MA, Wehr TA. A longer biological night in long sleepers than in short sleepers. J Clin Endocrinol Metab. 2003;88:26–30. 10.1210/jc.2002-020827. - DOI - PubMed
-
- Al-Ghoul WM, Herman MD, Dubocovich ML. Melatonin receptor subtype expression in human cerebellum. Neuroreport. 1998;9:4063–4068. - PubMed
-
- Arendt J. Melatonin, circadian rhythms, and sleep. N Engl J Med. 2000;343:1114–1116. 10.1056/NEJM200010123431510. - DOI - PubMed
-
- Arendt J, Bojkowski C, Folkard S, Franey C, Marks V, Minors D, Waterhouse J, Wever RA, Wildgruber C, Wright J. Some effects of melatonin and the control of its secretion in humans. In: Evered D, Clark S, editors. Photoperiodism, Melatonin and the Pineal. Vol. 117. London: Pitman; 1985. pp. 266–283. Ciba Foundation Symposium. - PubMed
-
- Attenburrow MEJ, Dowling BA, Sargent PA, Sharpley AL, Cowen PJ. Melatonin phase advances circadian rhythm. Psychopharmacology (Berl) 1995;121:503–505. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

