Mediation of Af4 protein function in the cerebellum by Siah proteins
- PMID: 15459319
- PMCID: PMC522018
- DOI: 10.1073/pnas.0406196101
Mediation of Af4 protein function in the cerebellum by Siah proteins
Abstract
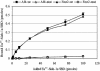
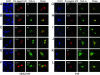
We have established that the gene AF4, which had long been recognized as disrupted in childhood leukemia, also plays a role in the CNS. Af4 is mutated in the robotic mouse that is characterized by ataxia and Purkinje cell loss. To determine the molecular basis of this mutation, we carried out a yeast two-hybrid screen and show that Af4 binds the E3 ubiquitin ligases Drosophila seven in absentia (sina) homologues (Siah)-1a and Siah-2 in the brain. Siah-1a and Af4 are expressed in Purkinje cells and colocalize in the nucleus of human embryonic kidney 293T and P19 cells. In vitro binding assays and coimmunoprecipitation reveal a significant reduction in affinity between Siah-1a and robotic mutant Af4 compared with wild-type, which correlates with the almost complete abolition of mutant Af4 degradation by Siah-1a. These data strongly suggest that an accumulation of mutant Af4 occurs in the robotic mouse due to a reduction in its normal turnover by the proteasome. A significant increase in the transcriptional activity of mutant Af4 relative to wild-type was obtained in mammalian cells, suggesting that the activity of Af4 is controlled through Siah-mediated degradation. Another member of the Af4 family, Fmr2, which is involved in mental handicap in humans, binds Siah proteins in a similar manner. These results provide evidence that a common regulatory mechanism exists that controls levels of the Af4/Fmr2 protein family. The robotic mouse thus provides a unique opportunity to understand how these proteins play a role in disorders as diverse as leukemia, mental retardation, and neurodegenerative disease.
Figures





References
-
- Heintz, N. & Zoghbi, H. (2000) Annu. Rev. Physiol. 62, 779-802. - PubMed
-
- Patil, N., Cox, D. R., Bhat, D., Faham, M., Myers, R. M. & Peterson, A. S. (1995) Nat. Genet. 2, 126-129. - PubMed
-
- Zuo, J., De Jager, P. L., Takahashi, K. A., Jiang, W., Linden, D. J. & Heintz, N. (1997) Nature 388, 769-773. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

