Promiscuous protein biotinylation by Escherichia coli biotin protein ligase
- PMID: 15459338
- PMCID: PMC2286582
- DOI: 10.1110/ps.04911804
Promiscuous protein biotinylation by Escherichia coli biotin protein ligase
Abstract
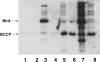
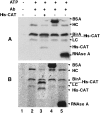
Biotin protein ligases (BPLs) are enzymes of extraordinary specificity. BirA, the BPL of Escherichia coli biotinylates only a single cellular protein. We report a mutant BirA that attaches biotin to a large number of cellular proteins in vivo and to bovine serum albumin, chloramphenicol acetyltransferase, immunoglobin heavy and light chains, and RNAse A in vitro. The mutant BirA also self biotinylates in vivo and in vitro. The wild type BirA protein is much less active in these reactions. The biotinylation reaction is proximity-dependent in that a greater extent of biotinylation was seen when the mutant ligase was coupled to the acceptor proteins than when the acceptors were free in solution. This approach may permit facile detection and recovery of interacting proteins by existing avidin/streptavidin technology.
Figures




References
-
- Barker, D.F. and Campbell, A.M. 1981. Genetic and biochemical characterization of the birA gene and its product: Evidence for a direct role of biotin holoenzyme synthetase in repression of the biotin operon in Escherichia coli. J. Mol. Biol. 146 469–492. - PubMed
-
- Beckett, D. and Matthews, B.W. 1997. Escherichia coli repressor of biotin biosynthesis. Methods Enzymol. 279 362–376. - PubMed
-
- Brown, P.H., Cronan, J.E., Grotli, M., and Beckett, D. 2004. The biotin repressor: Modulation of allostery by corepressor analogs. J. Mol. Biol. 337 857–869. - PubMed
-
- Chapman-Smith, A. and Cronan Jr., J.E. 1999. The enzymatic biotinylation of proteins: A post-translational modification of exceptional specificity. Trends Biochem. Sci. 24 359–363. - PubMed
-
- Chapman-Smith, A., Morris, T.W., Wallace, J.C., and Cronan Jr., J.E. 1999. Molecular recognition in a post-translational modification of exceptional specificity. Mutants of the biotinylated domain of acetyl-CoA carboxylase defective in recognition by biotin protein ligase. J. Biol. Chem. 274 1449–1457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

