Filtering of calcium transients by the endoplasmic reticulum in pancreatic beta-cells
- PMID: 15465863
- PMCID: PMC1304890
- DOI: 10.1529/biophysj.104.050955
Filtering of calcium transients by the endoplasmic reticulum in pancreatic beta-cells
Abstract
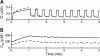
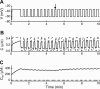
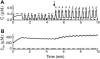
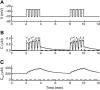
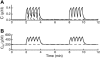
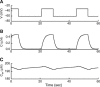
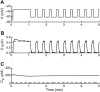
Calcium handling in pancreatic beta-cells is important for intracellular signaling, the control of electrical activity, and insulin secretion. The endoplasmic reticulum (ER) is a key organelle involved in the storage and release of intracellular Ca2+. Using mathematical modeling, we analyze the filtering properties of the ER and clarify the dual role that it plays as both a Ca2+ source and a Ca2+ sink. We demonstrate that recent time-dependent data on the free Ca2+ concentration in pancreatic islets and beta-cell clusters can be explained with a model that uses a passive ER that takes up Ca2+ when the cell is depolarized and the cytosolic Ca2+ concentration is elevated, and releases Ca2+ when the cell is repolarized and the cytosolic Ca2+ is at a lower concentration. We find that Ca2+-induced Ca2+ release is not necessary to explain the data, and indeed the model is inconsistent with the data if Ca2+-induced Ca2+ release is a dominating factor. Finally, we show that a three-compartment model that includes a subspace compartment between the ER and the plasma membrane provides the best agreement with the experimental Ca2+ data.
Figures




References
-
- Ämmälä, C., P.-O. Larsson, P.-O. Berggren, K. Bokvist, L. Juntti-Berggren, H. Kindmark, and P. Rorsman. 1991. Inositol trisphosphate-dependent periodic activation of a Ca2+-activated K+ conductance in glucose-stimulated pancreatic β-cells. Nature. 353:849–852. - PubMed
-
- Arredouani, A., Y. Guiot, J.-C. Jonas, L. H. Liu, M. Nenquin, J. A. Pertusa, J. Rahier, J.-F. Rolland, G. E. Shull, M. Stevens, F. Wuytack, J. C. Henquin, and P. Gilon. 2002a. SERCA3 ablation does not impair insulin secretion but suggests distinct roles of different sarcoendoplasmic reticulum Ca2+ pumps for Ca2+ homeostasis in pancreatic β-cells. Diabetes. 51:3245–3253. - PubMed
-
- Arredouani, A., J.-C. Henquin, and P. Gilon. 2002b. Contribution of the endoplasmic reticulum to the glucose-induced [Ca2+]c response in mouse pancreatic islets. Am. J. Physiol. 282:E982–E991. - PubMed
-
- Ashcroft, F. M., and P. Rorsman. 1989. Electrophysiology of the pancreatic β-cell. Prog. Biophys. Mol. Biol. 54:87–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

