Conditional mutagenesis using site-specific recombination in Plasmodium berghei
- PMID: 15465918
- PMCID: PMC522007
- DOI: 10.1073/pnas.0404416101
Conditional mutagenesis using site-specific recombination in Plasmodium berghei
Abstract
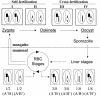
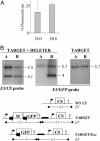
Reverse genetics in Plasmodium, the genus of parasites that cause malaria, still faces major limitations. Only red blood cell stages of this haploid parasite can be transfected. Consequently, the function of many essential genes in these and subsequent stages, including those encoding vaccine candidates, cannot be addressed genetically. Here, we establish conditional mutagenesis in Plasmodium by using site-specific recombination and the Flp/FRT system of yeast. Site-specific recombination is induced after cross-fertilization in the mosquito vector of two clones containing either the target sequence flanked by two FRT sites or the Flp recombinase. Parasites that have undergone recombination are recognized in the cross progeny through the expression of a fluorescence marker. This approach should permit to dissect the function of any essential gene of Plasmodium during the haploid phase of its life, i.e., during infection of salivary glands in the mosquito and infection of both the liver and red blood cells in the mammal.
Figures

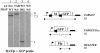
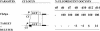

Similar articles
-
Clonal conditional mutagenesis in malaria parasites.Cell Host Microbe. 2009 Apr 23;5(4):386-96. doi: 10.1016/j.chom.2009.03.008. Cell Host Microbe. 2009. PMID: 19380117
-
FLP/FRT-mediated conditional mutagenesis in pre-erythrocytic stages of Plasmodium berghei.Nat Protoc. 2011 Aug 25;6(9):1412-28. doi: 10.1038/nprot.2011.363. Nat Protoc. 2011. PMID: 21886105
-
Conditional Gene Deletion in Mammalian and Mosquito Stages of Plasmodium berghei Using Dimerizable Cre Recombinase.Methods Mol Biol. 2021;2369:101-120. doi: 10.1007/978-1-0716-1681-9_7. Methods Mol Biol. 2021. PMID: 34313986
-
Towards genome-wide experimental genetics in the in vivo malaria model parasite Plasmodium berghei.Pathog Glob Health. 2015 Mar;109(2):46-60. doi: 10.1179/2047773215Y.0000000006. Epub 2015 Mar 19. Pathog Glob Health. 2015. PMID: 25789828 Free PMC article. Review.
-
Fertilization is a novel attacking site for the transmission blocking of malaria parasites.Acta Trop. 2010 Jun;114(3):157-61. doi: 10.1016/j.actatropica.2009.08.005. Epub 2009 Aug 8. Acta Trop. 2010. PMID: 19665985 Review.
Cited by
-
A set of glycosylphosphatidyl inositol-anchored membrane proteins of Plasmodium falciparum is refractory to genetic deletion.Infect Immun. 2006 Jul;74(7):4330-8. doi: 10.1128/IAI.00054-06. Infect Immun. 2006. PMID: 16790807 Free PMC article.
-
Identification and characterization of a liver stage-specific promoter region of the malaria parasite Plasmodium.PLoS One. 2010 Oct 27;5(10):e13653. doi: 10.1371/journal.pone.0013653. PLoS One. 2010. PMID: 21048918 Free PMC article.
-
Using green fluorescent malaria parasites to screen for permissive vector mosquitoes.Malar J. 2006 Mar 28;5:23. doi: 10.1186/1475-2875-5-23. Malar J. 2006. PMID: 16569221 Free PMC article.
-
The malarial serine protease SUB1 plays an essential role in parasite liver stage development.PLoS Pathog. 2013;9(12):e1003811. doi: 10.1371/journal.ppat.1003811. Epub 2013 Dec 12. PLoS Pathog. 2013. PMID: 24348254 Free PMC article.
-
Advances in molecular genetic systems in malaria.Nat Rev Microbiol. 2015 Jun;13(6):373-87. doi: 10.1038/nrmicro3450. Nat Rev Microbiol. 2015. PMID: 25978707 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

