Replicon-specific regulation of small heat shock genes in Agrobacterium tumefaciens
- PMID: 15466035
- PMCID: PMC522190
- DOI: 10.1128/JB.186.20.6824-6829.2004
Replicon-specific regulation of small heat shock genes in Agrobacterium tumefaciens
Abstract
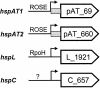
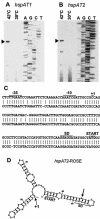
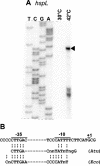
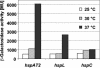
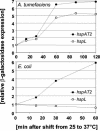
Four genes coding for small heat shock proteins (sHsps) were identified in the genome sequence of Agrobacterium tumefaciens, one on the circular chromosome (hspC), one on the linear chromosome (hspL), and two on the pAT plasmid (hspAT1 and hspAT2). Induction of sHsps at elevated temperatures was revealed by immunoblot analyses. Primer extension experiments and translational lacZ fusions demonstrated that expression of the pAT-derived genes and hspL is controlled by temperature in a regulon-specific manner. While the sHsp gene on the linear chromosome turned out to be regulated by RpoH (sigma32), both copies on pAT were under the control of highly conserved ROSE (named for repression of heat shock gene expression) sequences in their 5' untranslated region. Secondary structure predictions of the corresponding mRNA strongly suggest that it represses translation at low temperatures by masking the Shine-Dalgarno sequence. The hspC gene was barely expressed (if at all) and not temperature responsive.
Figures

References
-
- Babst, M., H. Hennecke, and H. M. Fischer. 1996. Two different mechanisms are involved in the heat-shock regulation of chaperonin gene expression in Bradyrhizobium japonicum. Mol. Microbiol. 19:827-839. - PubMed
-
- Chowdhury, S., C. Ragaz, E. Kreuger, and F. Narberhaus. 2003. Temperature-controlled structural alterations of an RNA thermometer. J. Biol. Chem. 278:47915-47921. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

