Biochemical characterization of the tobacco 42-kD protein kinase activated by osmotic stress
- PMID: 15466234
- PMCID: PMC523384
- DOI: 10.1104/pp.104.046151
Biochemical characterization of the tobacco 42-kD protein kinase activated by osmotic stress
Abstract
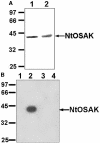
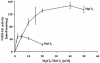
In tobacco (Nicotiana tabacum), hyperosmotic stress induces rapid activation of a 42-kD protein kinase, referred to as Nicotiana tabacum osmotic stress-activated protein kinase (NtOSAK). cDNA encoding the kinase was cloned and, based on the predicted amino acid sequence, the enzyme was assigned to the SNF1-related protein kinase type 2 (SnRK2) family. The identity of the enzyme was confirmed by immunoprecipitation of the active kinase from tobacco cells subjected to osmotic stress using antibodies raised against a peptide corresponding to the C-terminal sequence of the kinase predicted from the cloned cDNA. A detailed biochemical characterization of NtOSAK purified from stressed tobacco cells was performed. Our results show that NtOSAK is a calcium-independent Ser/Thr protein kinase. The sequence of putative phosphorylation sites recognized by NtOSAK, predicted by the computer program PREDIKIN, resembled the substrate consensus sequence defined for animal and yeast (Saccharomyces cerevisiae) AMPK/SNF1 kinases. Our experimental data confirmed these results, as various targets for AMPK/SNF1 kinases were also efficiently phosphorylated by NtOSAK. A range of protein kinase inhibitors was tested as potential modulators of NtOSAK, but only staurosporine, a rather nonspecific protein kinase inhibitor, was found to abolish the enzyme activity. In phosphorylation reactions, NtOSAK exhibited a preference for Mg(2+) over Mn(2+) ions and an inability to use GTP instead of ATP as a phosphate donor. The enzyme activity was not modulated by 5'-AMP. To our knowledge, these results represent the first detailed biochemical characterization of a kinase of the SnRK2 family.
Figures




References
-
- Carling D, Zammit VA, Hardie DG (1987) A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett 223: 217–222 - PubMed
-
- Chinnusamy V, Schumaker K, Zhu J-K (2004) Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J Exp Bot 55: 225–236 - PubMed
-
- Cohen P (1999) The development and therapeutic potential of protein kinase inhibitors. Curr Opin Chem Biol 3: 459–465 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

