A novel meiotic drive locus almost completely distorts segregation in mimulus (monkeyflower) hybrids
- PMID: 15466426
- PMCID: PMC1448871
- DOI: 10.1534/genetics.104.032789
A novel meiotic drive locus almost completely distorts segregation in mimulus (monkeyflower) hybrids
Abstract
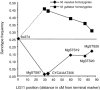
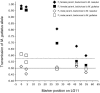
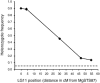
We report the discovery, mapping, and characterization of a meiotic drive locus (D) exhibiting nearly 100% nonrandom transmission in hybrids between two species of yellow monkeyflowers, outcrossing Mimulus guttatus and selfing M. nasutus. Only 1% of F(2) hybrids were M. nasutus homozygotes at the marker most tightly linked to D. We used a set of reciprocal backcrosses to distinguish among male-specific, female-specific, and zygote-specific sources of transmission ratio distortion. Transmission was severely distorted only when the heterozygous F(1) acted as the female parent in crosses to either parental species, ruling out pollen competition and zygote mortality as potential sources of drive. After four generations of backcrossing to M. nasutus, nearly isogenic lines were still >90% heterozygous at markers linked to D, suggesting that heterozygosity at the drive locus alone is sufficient for nonrandom transmission. A lack of dramatic female fitness costs in these lines rules out alternatives involving ovule or seed mortality and points to a truly meiotic mechanism of drive. The strength and direction of drive in this system is consistent with population genetic theory of selfish element evolution under different mating systems. These results are the first empirical demonstration of the strong female-specific drive predicted by new models of selfish centromere turnover.
Figures
References
-
- Burt, A., and R. Trivers, 1998. Selfish DNA and breeding system in flowering plants. Proc. R. Soc. Lond. Ser. B 265: 141–146.
-
- Dawe, R. K., 1998. Meiotic chromosome organization and segregation in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49: 371–395. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

