XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum
- PMID: 15466483
- PMCID: PMC2172532
- DOI: 10.1083/jcb.200406136
XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum
Abstract
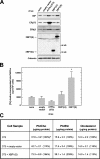
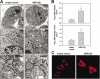
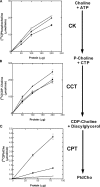
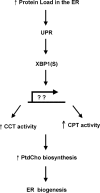
When the protein folding capacity of the endoplasmic reticulum (ER) is challenged, the unfolded protein response (UPR) maintains ER homeostasis by regulating protein synthesis and enhancing expression of resident ER proteins that facilitate protein maturation and degradation. Here, we report that enforced expression of XBP1(S), the active form of the XBP1 transcription factor generated by UPR-mediated splicing of XBP1 mRNA, is sufficient to induce synthesis of phosphatidylcholine, the primary phospholipid of the ER membrane. Cells overexpressing XBP1(S) exhibit elevated levels of membrane phospholipids, increased surface area and volume of rough ER, and enhanced activity of the cytidine diphosphocholine pathway of phosphatidylcholine biosynthesis. These data suggest that XBP1(S) links the mammalian UPR to phospholipid biosynthesis and ER biogenesis.
Figures

Comment in
-
Membrane biogenesis and the unfolded protein response.J Cell Biol. 2004 Oct 11;167(1):23-5. doi: 10.1083/jcb.200408117. J Cell Biol. 2004. PMID: 15479733 Free PMC article.
References
-
- Bligh, E.G., and W.J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Med. Sci. 37:911–917. - PubMed
-
- Calfon, M., H. Zeng, F. Urano, J.H. Till, S.R. Hubbard, H.P. Harding, S.G. Clark, and D. Ron. 2002. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 415:92–96. - PubMed
-
- Cornell, R.B., and I.C. Northwood. 2000. Regulation of CTP:phosphocholine cytidylyltransferase by amphitropism and relocalization. Trends Biochem. Sci. 25:441–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

