Stratified growth in Pseudomonas aeruginosa biofilms
- PMID: 15466566
- PMCID: PMC522130
- DOI: 10.1128/AEM.70.10.6188-6196.2004
Stratified growth in Pseudomonas aeruginosa biofilms
Abstract
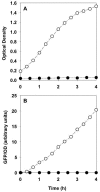
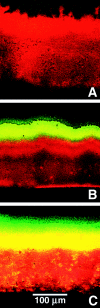
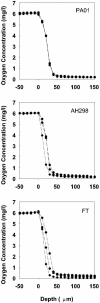
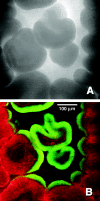
In this study, stratified patterns of protein synthesis and growth were demonstrated in Pseudomonas aeruginosa biofilms. Spatial patterns of protein synthetic activity inside biofilms were characterized by the use of two green fluorescent protein (GFP) reporter gene constructs. One construct carried an isopropyl-beta-d-thiogalactopyranoside (IPTG)-inducible gfpmut2 gene encoding a stable GFP. The second construct carried a GFP derivative, gfp-AGA, encoding an unstable GFP under the control of the growth-rate-dependent rrnBp(1) promoter. Both GFP reporters indicated that active protein synthesis was restricted to a narrow band in the part of the biofilm adjacent to the source of oxygen. The zone of active GFP expression was approximately 60 microm wide in colony biofilms and 30 microm wide in flow cell biofilms. The region of the biofilm in which cells were capable of elongation was mapped by treating colony biofilms with carbenicillin, which blocks cell division, and then measuring individual cell lengths by transmission electron microscopy. Cell elongation was localized at the air interface of the biofilm. The heterogeneous anabolic patterns measured inside these biofilms were likely a result of oxygen limitation in the biofilm. Oxygen microelectrode measurements showed that oxygen only penetrated approximately 50 microm into the biofilm. P. aeruginosa was incapable of anaerobic growth in the medium used for this investigation. These results show that while mature P. aeruginosa biofilms contain active, growing cells, they can also harbor large numbers of cells that are inactive and not growing.
Figures




References
-
- Cormack, B. P., R. H. Valdivia, and S. Falkow. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33-38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

