Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes
- PMID: 15466619
- PMCID: PMC2213293
- DOI: 10.1084/jem.20041236
Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes
Abstract
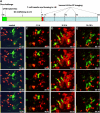
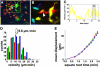
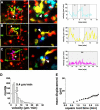
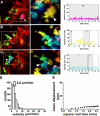
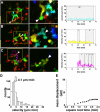
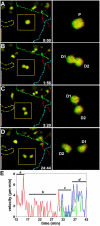
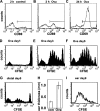
The adaptive immune response is initiated in secondary lymphoid organs by contact between antigen-bearing dendritic cells (DCs) and antigen-specific CD4+ T cells. However, there is scant information regarding the single cell dynamics of this process in vivo. Using two-photon microscopy, we imaged the real-time behavior of naive CD4+ T cells and in vivo-labeled DCs in lymph nodes during a robust T cell response. In the first 2 h after entry into lymph nodes, T cells made short-lived contacts with antigen-bearing DCs, each contact lasting an average of 11-12 min and occurring mainly on dendrites. Altered patterns of T cell motility during this early stage of antigen recognition promoted serial engagement with several adjacent DCs. Subsequently, T cell behavior progressed through additional distinct stages, including long-lived clusters, dynamic swarms, and finally autonomous migration punctuated by cell division. These observations suggest that the immunological synapse in native tissues is remarkably fluid, and that stable synapses form only at specific stages of antigen presentation to T cells. Furthermore, the serial nature of these interactions implies that T cells activate by way of multiple antigen recognition events.
Figures
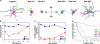

References
-
- Mellman, I., and R.M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell. 106:255–258. - PubMed
-
- Jenkins, M.K., A. Khoruts, E. Ingulli, D.L. Mueller, S.J. McSorley, R.L. Reinhardt, A. Itano, and K.A. Pape. 2001. In vivo activation of antigen-specific CD4 T cells. Annu. Rev. Immunol. 19:23–45. - PubMed
-
- Germain, R.N., and M.K. Jenkins. 2004. In vivo antigen presentation. Curr. Opin. Immunol. 16:120–125. - PubMed
-
- Dustin, M.L., and J.A. Cooper. 2000. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat. Immunol. 1:23–29. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

