GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells
- PMID: 15466621
- PMCID: PMC2213282
- DOI: 10.1084/jem.20031556
GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells
Abstract
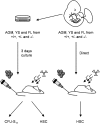
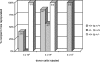
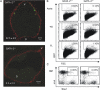
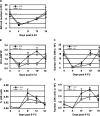
GATA-2 is an essential transcription factor in the hematopoietic system that is expressed in hematopoietic stem cells (HSCs) and progenitors. Complete deficiency of GATA-2 in the mouse leads to severe anemia and embryonic lethality. The role of GATA-2 and dosage effects of this transcription factor in HSC development within the embryo and adult are largely unexplored. Here we examined the effects of GATA-2 gene dosage on the generation and expansion of HSCs in several hematopoietic sites throughout mouse development. We show that a haploid dose of GATA-2 severely reduces production and expansion of HSCs specifically in the aorta-gonad-mesonephros region (which autonomously generates the first HSCs), whereas quantitative reduction of HSCs is minimal or unchanged in yolk sac, fetal liver, and adult bone marrow. However, HSCs in all these ontogenically distinct anatomical sites are qualitatively defective in serial or competitive transplantation assays. Also, cytotoxic drug-induced regeneration studies show a clear GATA-2 dose-related proliferation defect in adult bone marrow. Thus, GATA-2 plays at least two functionally distinct roles during ontogeny of HSCs: the production and expansion of HSCs in the aorta-gonad-mesonephros and the proliferation of HSCs in the adult bone marrow.
Figures


References
-
- Spangrude, G.J., L. Smith, N. Uchida, K. Ikuta, S. Heimfeld, J. Friedman, and I.L. Weissman. 1991. Mouse hematopoietic stem cells. Blood. 78:1395–1402. - PubMed
-
- Lemischka, I.R. 1991. Clonal, in vivo behavior of the totipotent hematopoietic stem cell. Semin. Immunol. 3:349–355. - PubMed
-
- Medvinsky, A., and E. Dzierzak. 1996. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 86:897–906. - PubMed
-
- Muller, A.M., A. Medvinsky, J. Strouboulis, F. Grosveld, and E. Dzierzak. 1994. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1:291–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

