Bruton's tyrosine kinase is essential for human B cell tolerance
- PMID: 15466623
- PMCID: PMC2213290
- DOI: 10.1084/jem.20040920
Bruton's tyrosine kinase is essential for human B cell tolerance
Abstract
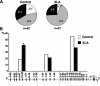
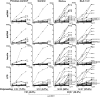
Most polyreactive and antinuclear antibodies are removed from the human antibody repertoire during B cell development. To elucidate how B cell receptor (BCR) signaling may regulate human B cell tolerance, we tested the specificity of recombinant antibodies from single peripheral B cells isolated from patients suffering from X-linked agammaglobulinemia (XLA). These patients carry mutations in the Bruton's tyrosine kinase (BTK) gene that encode an essential BCR signaling component. We find that in the absence of Btk, peripheral B cells show a distinct antibody repertoire consistent with extensive secondary V(D)J recombination. Nevertheless, XLA B cells are enriched in autoreactive clones. Our results demonstrate that Btk is essential in regulating thresholds for human B cell tolerance.
Figures

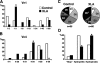


References
-
- Wardemann, H., S. Yurasov, A. Schaefer, J.W. Young, E. Meffre, and M.C. Nussenzweig. 2003. Predominant autoantibody production by early human B cell precursors. Science. 301:1374–1377. - PubMed
-
- Nemazee, D.A., and K. Bürki. 1989. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 337:562–566. - PubMed
-
- Goodnow, C.C., J. Crosbie, S. Adelstein, T.B. Lavoie, S.J. Smith-Gill, R.A. Brink, H. Pritchard-Briscoe, J.S. Wotherspoon, R.H. Loblay, K. Raphael, et al. 1988. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 334:676–682. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

