The complete genomic sequence of Nocardia farcinica IFM 10152
- PMID: 15466710
- PMCID: PMC522048
- DOI: 10.1073/pnas.0406410101
The complete genomic sequence of Nocardia farcinica IFM 10152
Abstract
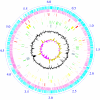
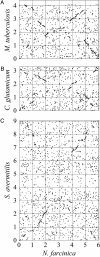
We determined the genomic sequence of Nocardia farcinica IFM 10152, a clinical isolate, and revealed the molecular basis of its versatility. The genome consists of a single circular chromosome of 6,021,225 bp with an average G+C content of 70.8% and two plasmids of 184,027 (pNF1) and 87,093 (pNF2) bp with average G+C contents of 67.2% and 68.4%, respectively. The chromosome encoded 5,674 putative protein-coding sequences, including many candidate genes for virulence and multidrug resistance as well as secondary metabolism. Analyses of paralogous protein families suggest that gene duplications have resulted in a bacterium that can survive not only in soil environments but also in animal tissues, resulting in disease.
Figures



Similar articles
-
Analysis of the complete genome sequence of Nocardia seriolae UTF1, the causative agent of fish nocardiosis: The first reference genome sequence of the fish pathogenic Nocardia species.PLoS One. 2017 Mar 3;12(3):e0173198. doi: 10.1371/journal.pone.0173198. eCollection 2017. PLoS One. 2017. PMID: 28257489 Free PMC article.
-
Contribution of rpoB2 RNA polymerase beta subunit gene to rifampin resistance in Nocardia species.Antimicrob Agents Chemother. 2006 Apr;50(4):1342-6. doi: 10.1128/AAC.50.4.1342-1346.2006. Antimicrob Agents Chemother. 2006. PMID: 16569850 Free PMC article.
-
Complete genome sequence analysis of Nocardia brasiliensis HUJEG-1 reveals a saprobic lifestyle and the genes needed for human pathogenesis.PLoS One. 2013 Jun 3;8(6):e65425. doi: 10.1371/journal.pone.0065425. Print 2013. PLoS One. 2013. PMID: 23755230 Free PMC article.
-
[Recent progress in taxonomic studies on pathogenic nocardia and usefulness of the bacteria for the studies on secondary metabolites and antibiotic resistant mechanisms].Nihon Ishinkin Gakkai Zasshi. 2010;51(4):179-92. doi: 10.3314/jjmm.51.179. Nihon Ishinkin Gakkai Zasshi. 2010. PMID: 21060211 Review. Japanese.
-
Borrelia genomes in the year 2000.J Mol Microbiol Biotechnol. 2000 Oct;2(4):401-10. J Mol Microbiol Biotechnol. 2000. PMID: 11075912 Review.
Cited by
-
The Nocardia cyriacigeorgica GUH-2 genome shows ongoing adaptation of an environmental Actinobacteria to a pathogen's lifestyle.BMC Genomics. 2013 Apr 27;14:286. doi: 10.1186/1471-2164-14-286. BMC Genomics. 2013. PMID: 23622346 Free PMC article.
-
Genome sequence of the human- and animal-pathogenic strain Nocardia cyriacigeorgica GUH-2.J Bacteriol. 2012 Apr;194(8):2098-9. doi: 10.1128/JB.00161-12. J Bacteriol. 2012. PMID: 22461543 Free PMC article.
-
Small but sufficient: the Rhodococcus phage RRH1 has the smallest known Siphoviridae genome at 14.2 kilobases.J Virol. 2012 Jan;86(1):358-63. doi: 10.1128/JVI.05460-11. Epub 2011 Oct 19. J Virol. 2012. PMID: 22013058 Free PMC article.
-
Cloning and characterization of alpha-methylacyl coenzyme A racemase from Gordonia polyisoprenivorans VH2.Appl Environ Microbiol. 2008 Nov;74(22):7085-9. doi: 10.1128/AEM.01491-08. Epub 2008 Sep 26. Appl Environ Microbiol. 2008. PMID: 18820059 Free PMC article.
-
Genome analysis reveals three genomospecies in Mycobacterium abscessus.BMC Genomics. 2014 May 12;15(1):359. doi: 10.1186/1471-2164-15-359. BMC Genomics. 2014. PMID: 24886480 Free PMC article.
References
-
- Brown, J. M., McNeil, M. M. & Desmond, E. P. (1999) in Manual of Clinical Microbiology, eds. Murray, P. R., Baron, E. J., Pfaller, M. A., Tenover, F. C. & Yolken, R. H. (Am. Soc. Microbiol., Washington, DC), pp. 370-398.
-
- Kageyama, A., Yazawa, K., Ishikawa, J., Hotta, K., Nishimura, K. & Mikami, Y. (2004) Eur. J. Epidemiol. 19, 383-389. - PubMed
-
- Shigemori, H., Komaki, H., Yazawa, K., Mikami, Y., Nemoto, A., Tanaka, Y., Sasaki, Y., Ishida, T. & Kobayashi, J. (1998) J. Org. Chem. 63, 6900-6904. - PubMed
-
- Tanaka, Y., Komaki, H., Yazawa, K., Mikami, Y., Nemoto, A., Tojyo, T., Kadowaki, K., Shigemori, H. & Kobayashi, J. (1997) J. Antibiot. 50, 1036-1041. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

