Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass
- PMID: 15467835
- PMCID: PMC518666
- DOI: 10.1172/JCI22098
Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass
Abstract
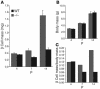
The endocrine pancreas undergoes major remodeling during neonatal development when replication of differentiated beta cells is the major mechanism by which beta cell mass is regulated. The molecular mechanisms that govern the replication of terminally differentiated beta cells are unclear. We show that during neonatal development, cyclin D2 expression in the endocrine pancreas coincides with the replication of endocrine cells and a massive increase in islet mass. Using cyclin D2-/- mice, we demonstrate that cyclin D2 is required for the replication of endocrine cells but is expendable for exocrine and ductal cell replication. As a result, 14-day-old cyclin D2-/- mice display dramatically smaller islets and a 4-fold reduction in beta cell mass in comparison to their WT littermates. Consistent with these morphological findings, the cyclin D2-/- mice are glucose intolerant. These results suggest that cyclin D2 plays a key role in regulating the transition of beta cells from quiescence to replication and may provide a target for the development of therapeutic strategies to induce expansion and/or regeneration of beta cells.
Figures
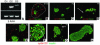
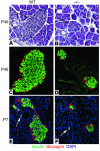
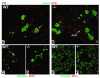
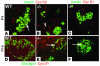
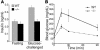
References
-
- Bonner-Weir S. Islet growth and development in the adult. J. Mol. Endocrinol. 2000;24:297–302. - PubMed
-
- Lingohr MK, Buettner R, Rhodes CJ. Pancreatic beta-cell growth and survival--a role in obesity-linked type 2 diabetes? [review] Trends Mol. Med. 2002;8:375–384. - PubMed
-
- Butler AE, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

