Costimulation controls diabetes by altering the balance of pathogenic and regulatory T cells
- PMID: 15467837
- PMCID: PMC518661
- DOI: 10.1172/JCI20483
Costimulation controls diabetes by altering the balance of pathogenic and regulatory T cells
Abstract
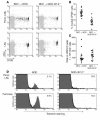
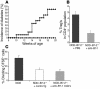
The development of autoimmune diabetes in the nonobese diabetic (NOD) mouse results from a breakdown in tolerance to pancreatic islet antigens. CD28-B7 and CD40 ligand-CD40 (CD40L-CD40) costimulatory pathways affect the development of disease and are promising therapeutic targets. Indeed, it was shown previously that diabetes fails to develop in NOD-B7-2-/- and NOD-CD40L-/- mice. In this study, we examined the relative role of these 2 costimulatory pathways in the balance of autoimmunity versus regulation in NOD mice. We demonstrate that initiation but not effector function of autoreactive T cells was defective in NOD-B7-2-/- mice. Moreover, the residual proliferation of the autoreactive cells was effectively controlled by CD28-dependent CD4+CD25+ regulatory T cells (Treg's), as depletion of Treg's partially restored proliferation of autoreactive T cells and resulted in diabetes in an adoptive-transfer model. Similarly, disruption of the CD28-B7 pathway and subsequent Treg deletion restored autoimmunity in NOD-CD40L-/- mice. These results demonstrate that development of diabetes is dependent on a balance of pathogenic and regulatory T cells that is controlled by costimulatory signals. Thus, elimination of Treg's results in diabetes even in the absence of costimulation, which suggests a need for alternative strategies for immunotherapeutic approaches.
Figures




References
-
- Delovitch TL, Singh B. The nonobese diabetic mouse as a model of autoimmune diabetes: immune dysregulation gets the NOD. Immunity. 1997;7:727–738. - PubMed
-
- Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 2001;19:225–252. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

