Contractions of D4Z4 on 4qB subtelomeres do not cause facioscapulohumeral muscular dystrophy
- PMID: 15467981
- PMCID: PMC1182148
- DOI: 10.1086/426035
Contractions of D4Z4 on 4qB subtelomeres do not cause facioscapulohumeral muscular dystrophy
Abstract
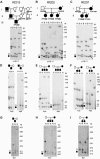
Facioscapulohumeral muscular dystrophy (FSHD) is associated with contractions of the D4Z4 repeat in the subtelomere of chromosome 4q. Two allelic variants of chromosome 4q (4qA and 4qB) exist in the region distal to D4Z4. Although both variants are almost equally frequent in the population, FSHD is associated exclusively with the 4qA allele. We identified three families with FSHD in which each proband carries two FSHD-sized alleles and is heterozygous for the 4qA/4qB polymorphism. Segregation analysis demonstrated that FSHD-sized 4qB alleles are not associated with disease, since these were present in unaffected family members. Thus, in addition to a contraction of D4Z4, additional cis-acting elements on 4qA may be required for the development of FSHD. Alternatively, 4qB subtelomeres may contain elements that prevent FSHD pathogenesis.
Figures
References
Electronic-Database Information
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for FSHD1A) - PubMed
References
-
- Arahata K, Ishihara T, Fukunaga H, Orimo S, Lee JH, Goto K, Nonaka I (1995) Inflammatory response in facioscapulohumeral muscular dystrophy (FSHD): immunocytochemical and genetic analyses. Muscle Nerve 2:S56–S66 - PubMed
-
- Auer-Grumbach M, Wagner K, Strasser-Fuchs S, Loscher WN, Fazekas F, Millner M, Hartung HP (2000) Clinical predominance of proximal upper limb weakness in CMT1A syndrome. Muscle Nerve 23:1243–1249 - PubMed
-
- Bakker E, Wijmenga C, Vossen RH, Padberg GW, Hewitt J, van der Wielen M, Rasmussen K, Frants RR (1995) The FSHD-linked locus D4F104S1 (p13E-11) on 4q35 has a homologue on 10qter. Muscle Nerve 2:S39–S44 - PubMed
-
- Baldwin CT, Hoth CF, Amos JA, Dasilva EO, Milunsky A (1992) An exonic mutation in the Hup2 paired domain gene causes Waardenburg syndrome. Nature 355:637–638 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

