Short tetracysteine tags to beta-tubulin demonstrate the significance of small labels for live cell imaging
- PMID: 15469986
- PMCID: PMC532040
- DOI: 10.1091/mbc.e04-06-0454
Short tetracysteine tags to beta-tubulin demonstrate the significance of small labels for live cell imaging
Abstract
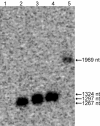
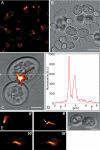
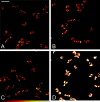
Genetically encoded tags are of fundamental importance for live cell imaging. We show that small tetracysteine (TetCys) tags can be highly advantageous for the functionality of the host protein compared with large fluorescent protein tags. One to three concatenated small TetCys tags as well as the large green fluorescent protein (GFP) were fused by integrative epitope tagging to the C terminus of beta-tubulin (Tub2) in the budding yeast Saccharomyces cerevisiae. The increasing tag size correlated with functional interference to the host protein. Tub2 tagged with either 1 x TetCys (10 amino acids [aa]) or 2 x TetCys (20 aa) was able to substitute Tub2 in haploid cells. In contrast, C-terminal tagging of Tub2 with 3 x TetCys (29 aa) or with GFP (244 aa) resulted in nonviable haploid cells. Cells expressing Tub2-1 x TetCys or Tub2-2 x TetCys were stained with FlAsH, which selectively binds to the TetCys-tag. The stained cells displayed dynamic FlAsH-labeled microtubules and low cellular background fluorescence. The presented approach to tag open reading frames (ORFs) at their native loci with very small TetCys-tags and the subsequent visualization of the tagged proteins in vivo can be extended in principle to any ORF in S. cerevisiae.
Figures


References
-
- Adams, S.R., Campbell, R.E., Gross, L.A., Martin, B.R., Walkup, G.K., Yao, Y., Llopis, J., and Tsien, R.Y. (2002). New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: synthesis and biological applications. J. Am. Chem. Soc. 124, 6063-6076. - PubMed
-
- De Antoni, A., and Gallwitz, D. (2000). A novel multi-purpose cassette for repeated integrative epitope tagging of genes in Saccharomyces cerevisiae. Gene 246, 179-185. - PubMed
-
- Dolinski, K., et al. (2004). Saccharomyces Genome Database. http://www.yeastgenome.org/.
-
- Eggeling, C., Widengren, J., Rigler, R., and Seidel, C.A.M. (1999). Photostabilities of fluorescent dyes for single-molecule spectroscopy: mechanisms and experimental methods for estimating photobleaching in aqueous solution. In: Applied Fluorescence in Chemistry, Biology and Medicine, ed. W. Rettig, B. Strehmel, M. Schrader, and H. Seifert, Berlin: Springer, 193-240.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

