Rebuilding an immune-mediated central nervous system disease: weighing the pathogenicity of antigen-specific versus bystander T cells
- PMID: 15470017
- PMCID: PMC5319420
- DOI: 10.4049/jimmunol.173.8.4779
Rebuilding an immune-mediated central nervous system disease: weighing the pathogenicity of antigen-specific versus bystander T cells
Abstract
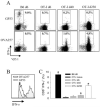
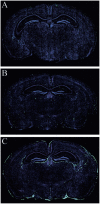
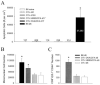
Although both self- and pathogen-specific T cells can participate in tissue destruction, recent studies have proposed that after viral infection, bystander T cells of an irrelevant specificity can bypass peptide-MHC restriction and contribute to undesired immunopathological consequences. To evaluate the importance of this mechanism of immunopathogenesis, we determined the relative contributions of Ag-specific and bystander CD8+ T cells to the development of CNS disease. Using lymphocytic choriomeningitis virus (LCMV) as a stimulus for T cell recruitment into the CNS, we demonstrate that bystander CD8+ T cells with an activated surface phenotype can indeed be recruited into the CNS over a chronic time window. These cells become anatomically positioned in the CNS parenchyma, and a fraction aberrantly acquires the capacity to produce the effector cytokine, IFN-gamma. However, when directly compared with their virus-specific counterparts, the contribution of bystander T cells to CNS damage was insignificant in nature (even when specifically activated). Although bystander T cells alone failed to cause tissue injury, transferring as few as 1000 naive LCMV-specific CD8+ T cells into a restricted repertoire containing only bystander T cells was sufficient to induce immune-mediated pathology and reconstitute a fatal CNS disease. These studies underscore the importance of specific T cells in the development of immunopathology and subsequent disease. Because of highly restrictive constraints imposed by the host, it is more likely that specific, rather than nonspecific, bystander T cells are the active participants in T cell-mediated diseases that afflict humans.
Figures







References
-
- Zinkernagel RM, Doherty PC. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974;248:701. - PubMed
-
- Stern LJ, Wiley DC. Antigenic peptide binding by class I and class II histocompatibility proteins. Structure. 1994;2:245. - PubMed
-
- Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947. - PubMed
-
- Gangappa S, Babu JS, Thomas J, Daheshia M, Rouse BT. Virus-induced immunoinflammatory lesions in the absence of viral antigen recognition. J. Immunol. 1998;161:4289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

