Characterization of the c-Jun N-terminal kinase-BimEL signaling pathway in neuronal apoptosis
- PMID: 15470142
- PMCID: PMC6729963
- DOI: 10.1523/JNEUROSCI.2953-04.2004
Characterization of the c-Jun N-terminal kinase-BimEL signaling pathway in neuronal apoptosis
Erratum in
- J Neurosci. 2004 Oct 27;24(43):1 p following 9722
Abstract
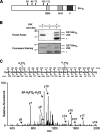
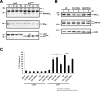
The c-Jun N-terminal kinase (JNK) signaling pathway plays a critical role in mediating apoptosis in the nervous system; however, the mechanisms by which JNK triggers neuronal apoptosis remain incompletely understood. Recent studies suggest that in addition to inducing transcription of pro-apoptotic genes, JNK also directly activates the cell death machinery. Here, we report that JNK catalyzed the phosphorylation of the BH3-only protein Bcl-2 interacting mediator of cell death (BimEL) at serine 65, both in vitro and in vivo. The JNK-induced phosphorylation of BimEL at serine 65 promoted the apoptotic effect of BimEL in primary cerebellar granule neurons. We also characterized the role of the JNK-BimEL signaling pathway in apoptosis that was triggered by overexpression of the p75 neurotrophin receptor (p75NTR). We found that activation of p75NTR induced the JNK-dependent phosphorylation of endogenous BimEL at serine 65 in cells. The genetic knockdown of BimEL by RNA interference or the expression of a dominant interfering form of BimEL significantly impaired the ability of activated p75NTR to induce apoptosis. Together, these results suggest that JNK-induced phosphorylation of BimEL at serine 65 mediates p75NTR-induced apoptosis. Our findings define a novel mechanism by which a death-receptor pathway directly activates the mitochondrial apoptotic machinery.
Figures



References
-
- Dechant G, Barde YA (2002) The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat Neurosci 5: 1131-1136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
