Distribution and injury-induced plasticity of cadherins in relationship to identified synaptic circuitry in adult rat spinal cord
- PMID: 15470146
- PMCID: PMC6729957
- DOI: 10.1523/JNEUROSCI.2726-04.2004
Distribution and injury-induced plasticity of cadherins in relationship to identified synaptic circuitry in adult rat spinal cord
Abstract
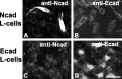
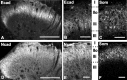
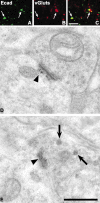
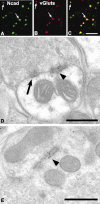
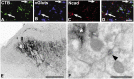
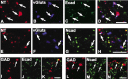
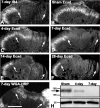
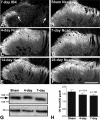
Cadherins are synaptically enriched cell adhesion and signaling molecules. In brain, they function in axon targeting and synaptic plasticity. In adult spinal cord, their localization, synaptic affiliation, and role in injury-related plasticity are mostly unexplored. Here, we demonstrate in adult rat dorsal horn that E- and N-cadherin display unique patterns of localization to functionally distinct types of synapses of intrinsic and primary afferent origin. Within the nociceptive afferent pathway to lamina II, nonpeptidergic C-fiber synapses in the deeper half of lamina II (IIi) contain E-cadherin but mostly lack N-cadherin, whereas the majority of the peptidergic C-fiber synapses in the outer half of lamina II (IIo) contain N-cadherin but lack E-cadherin. Approximately one-half of the Abeta-fiber terminations in lamina III contain N-cadherin; none contain E-cadherin. Strikingly, the distribution and levels of these cadherins are differentially affected by sciatic nerve axotomy, a model of neuropathic pain in which degenerative and regenerative structural plasticity has been implicated. Within the first 7 d after axotomy, E-cadherin is rapidly and completely lost from the dorsal horn synapses with which it is affiliated, whereas N-cadherin localization and levels are unchanged; such patterns persist through 28 d postlesion. The loss of E-cadherin thus occurs before the onset of mechanical hyperalgesia (approximately 10-21 d postlesion), as reported previously. Together, the synaptic specificity displayed by these cadherins, coupled with their differential response to injury, suggests that they may proactively contribute to the maintenance of some, and incipient dismantling of other, synaptic circuits in response to nerve injury. Speculatively, such changes may ultimately contribute to subsequently emerging abnormalities in pain perception.
Figures


References
-
- Alvarez FJ, Morris HR, Priestley JV (1991) Sub-populations of smaller diameter trigeminal primary afferent neurons defined by expression of calcitonin gene-related peptide and the cell surface oligosaccharide recognized by monoclonal antibody LA4. J Neurocytol 20: 716-731. - PubMed
-
- Arndt K, Nakagawa S, Takeichi M, Redies C (1998) Cadherin-defined segments and parasagittal cell ribbons in the developing chicken cerebellum. Mol Cell Neurosci 10: 211-228. - PubMed
-
- Arvidsson J, Ygge J, Grant G (1986) Cell loss in lumbar dorsal root ganglia and transganglionic degeneration after sciatic nerve resection in the rat. Brain Res 373: 15-21. - PubMed
-
- Bao L, Wang HF, Cai HJ, Tong YG, Jin SX, Lu YJ, Grant G, Hokfelt T, Zhang X (2002) Peripheral axotomy induces only very limited sprouting of coarse myelinated afferents into inner lamina II of rat spinal cord. Eur J Neurosci 16: 175-185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
