Rapid production of gene replacement constructs and generation of a green fluorescent protein-tagged centromeric marker in Aspergillus nidulans
- PMID: 15470263
- PMCID: PMC522605
- DOI: 10.1128/EC.3.5.1359-1362.2004
Rapid production of gene replacement constructs and generation of a green fluorescent protein-tagged centromeric marker in Aspergillus nidulans
Abstract
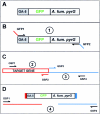
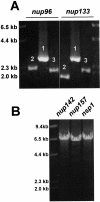
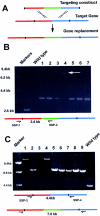
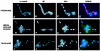
A method to rapidly generate gene replacement constructs by fusion PCR is described for Aspergillus nidulans. The utility of the approach is demonstrated by green fluorescent protein (GFP) tagging of A. nidulans ndc80 to visualize centromeres through the cell cycle. The methodology makes possible large-scale GFP tagging, promoter swapping, and deletion analysis of A. nidulans.
Figures
References
-
- Bussink, H. J., and S. A. Osmani. 1999. A mitogen-activated protein kinase (MPKA) is involved in polarized growth in the filamentous fungus, Aspergillus nidulans. FEMS Microbiol. Lett. 173:117-125. - PubMed
-
- Fernandez-Abalos, J. M., H. Fox, C. Pitt, B. Wells, and J. H. Doonan. 1998. Plant-adapted green fluorescent protein is a versatile vital reporter for gene expression, protein localization and mitosis in the filamentous fungus, Aspergillus nidulans. Mol. Microbiol. 27:121-130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

