The wrickkened pathways of FGF23, MEPE and PHEX
- PMID: 15470265
- PMCID: PMC3361894
- DOI: 10.1177/154411130401500503
The wrickkened pathways of FGF23, MEPE and PHEX
Abstract
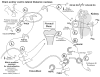
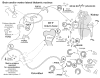
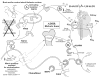
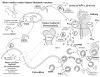
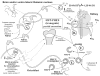
The last 350 years since the publication of the first medical monograph on rickets (old English term wrickken) (Glisson et al., 1651) have seen spectacular advances in our understanding of mineral-homeostasis. Seminal and exciting discoveries have revealed the roles of PTH, vitamin D, and calcitonin in regulating calcium and phosphate, and maintaining healthy teeth and skeleton. However, it is clear that the PTH/Vitamin D axis does not account for the entire picture, and a new bone-renal metabolic milieu has emerged, implicating a novel set of matrix proteins, hormones, and Zn-metallopeptidases. The primary defects in X-linked hypophosphatemic rickets (HYP) and autosomal-dominant hypophosphatemic rickets (ADHR) are now identified as inactivating mutations in a Zn-metalloendopeptidase (PHEX) and activating mutations in fibroblast-growth-factor-23 (FGF23), respectively. In oncogenic hypophosphatemic osteomalacia (OHO), several tumor-expressed proteins (MEPE, FGF23, and FRP-4) have emerged as candidate mediators of the bone-renal pathophysiology. This has stimulated the proposal of a global model that takes into account the remarkable similarities between the inherited diseases (HYP and ADHR) and the tumor-acquired disease OHO. In HYP, loss of PHEX function is proposed to result in an increase in uncleaved full-length FGF23 and/or inappropriate processing of MEPE. In ADHR, a mutation in FGF23 results in resistance to proteolysis by PHEX or other proteases and an increase in half-life of full-length phosphaturic FGF23. In OHO, over-expression of FGF23 and/or MEPE is proposed to result in abnormal renal-phosphate handling and mineralization. Although this model is attractive, many questions remain unanswered, suggesting a more complex picture. The following review will present a global hypothesis that attempts to explain the experimental and clinical observations in HYP, ADHR, and OHO, plus diverse mouse models that include the MEPE null mutant, HYP-PHEX transgenic mouse, and MEPE-PHEX double-null-mutant.
Figures

References
-
- Aisa MC, Rahman S, Senin U, Maggio D, Russell RG. Cathepsin B activity in normal human osteoblast-like cells and human osteoblastic osteosarcoma cells (MG-63): regulation by interleukin-1 beta and parathyroid hormone. Biochim Biophys Acta. 1996;1290:29–36. - PubMed
-
- Aisa MC, Beccari T, Costanzi E, Maggio D. Cathepsin B in osteoblasts. Biochim Biophys Acta. 2003;1621:149–159. - PubMed
-
- Allen HC, Fedarko NS, Jain A, Whybro A, Barker ME, Eastell R, et al. The response of MEPE to long-term and short-term phosphate supplementation in healthy volunteers (abstract) J Bone Miner Res. 2003;18:S116.
-
- Argiro L, Desbarats M, Glorieux FH, Ecarot B. Mepe, the gene encoding a tumor-secreted protein in oncogenic hypophosphatemic osteomalacia, is expressed in bone. Genomics. 2001;74:342–351. - PubMed
-
- Aschinberg LC, Solomon LM, Zeis PM, Justice P, Rosenthal IM. Vitamin D-resistant rickets associated with epidermal nevus syndrome: demonstration of a phosphaturic substance in the dermal lesions. J Pediatr. 1977;91:56–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
