A system for studying epithelial-stromal interactions reveals distinct inductive abilities of stromal cells from benign prostatic hyperplasia and prostate cancer
- PMID: 15471963
- PMCID: PMC3033046
- DOI: 10.1210/en.2004-1123
A system for studying epithelial-stromal interactions reveals distinct inductive abilities of stromal cells from benign prostatic hyperplasia and prostate cancer
Abstract
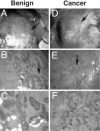
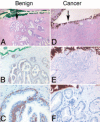
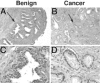
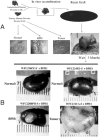
The development of normal and abnormal glandular structures in the prostate is controlled at the endocrine and paracrine levels by reciprocal interactions between epithelium and stroma. To study these processes, it is useful to have an efficient method of tissue acquisition for reproducible isolation of cells from defined histologies. Here we assessed the utility of a standardized system for acquisition and growth of prostatic cells from different regions of the prostate with different pathologies, and we compared the abilities of stromal cells from normal peripheral zone, benign prostatic hyperplasia (BPH-S), and cancer to induce the growth of a human prostatic epithelial cell line (BPH-1) in vivo. Using the tissue recombination method, we showed that grafting stromal cells (from any histology) alone or BPH-1 epithelial cells alone produced no visible grafts. Recombining stromal cells from normal peripheral zone with BPH-1 cells also produced no visible grafts (n = 15). Recombining BPH-S with BPH-1 cells generated small, well-organized, and sharply demarcated grafts approximately 3-4 mm in diameter (n = 9), demonstrating a moderate inductive ability of BPH-S. Recombining stromal cells from cancer with BPH-1 cells generated highly disorganized grafts that completely surrounded the host kidney and invaded into adjacent renal tissue, demonstrating induction of an aggressive phenotype. We conclude that acquisition of tissue from toluidine blue dye-stained specimens is an efficient method to generate high-quality epithelial and/or stromal cultures. Stromal cells derived by this method from areas of BPH and cancer induce epithelial cell growth in vivo, which mimics the natural history of these diseases.
Figures


Comment in
-
Are times a' changin' in carcinogenesis?Endocrinology. 2005 Jan;146(1):11-2. doi: 10.1210/en.2004-1376. Endocrinology. 2005. PMID: 15601901 Review. No abstract available.
References
-
- Cunha G. Epithelio-mesenchymal interaction in primordial gland structures which become responsive to androgenic stimulation. Anat Rec. 1972;172:179–196. - PubMed
-
- Cunha GR, Chung LW. Stromal-epithelial interactions—I. Induction of prostatic phenotype in urothelium of testicular feminized (Tfm/y) mice. J Steroid Biochem. 1981;14:1317–1324. - PubMed
-
- Cunha GR, Fujii H, Neubauer BL, Shannon JM, Sawyer L, Reese BA. Epithelial-mesenchymal interactions in prostatic development. I. morphological observations of prostatic induction by urogenital sinus mesenchyme in epithelium of the adult rodent urinary bladder. J Cell Biol. 1983;96:1662–1670. - PMC - PubMed
-
- Cunha GR. Androgenic effects upon prostatic epithelium are mediated via trophic influences from stroma. Prog Clin Biol Res. 1984;145:81–102. - PubMed
-
- Cunha G, Donjacour A, Cooke P, Mee S, Bigsby RM, Higgins SJ, Sugimura Y. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987;8:338–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

