Dorsal-ventral patterning and neural induction in Xenopus embryos
- PMID: 15473842
- PMCID: PMC2280069
- DOI: 10.1146/annurev.cellbio.20.011403.154124
Dorsal-ventral patterning and neural induction in Xenopus embryos
Abstract
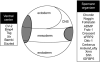
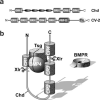
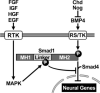
We review the current status of research in dorsal-ventral (D-V) patterning in vertebrates. Emphasis is placed on recent work on Xenopus, which provides a paradigm for vertebrate development based on a rich heritage of experimental embryology. D-V patterning starts much earlier than previously thought, under the influence of a dorsal nuclear -Catenin signal. At mid-blastula two signaling centers are present on the dorsal side: The prospective neuroectoderm expresses bone morphogenetic protein (BMP) antagonists, and the future dorsal endoderm secretes Nodal-related mesoderm-inducing factors. When dorsal mesoderm is formed at gastrula, a cocktail of growth factor antagonists is secreted by the Spemann organizer and further patterns the embryo. A ventral gastrula signaling center opposes the actions of the dorsal organizer, and another set of secreted antagonists is produced ventrally under the control of BMP4. The early dorsal -Catenin signal inhibits BMP expression at the transcriptional level and promotes expression of secreted BMP antagonists in the prospective central nervous system (CNS). In the absence of mesoderm, expression of Chordin and Noggin in ectoderm is required for anterior CNS formation. FGF (fibroblast growth factor) and IGF (insulin-like growth factor) signals are also potent neural inducers. Neural induction by anti-BMPs such as Chordin requires mitogen-activated protein kinase (MAPK) activation mediated by FGF and IGF. These multiple signals can be integrated at the level of Smad1. Phosphorylation by BMP receptor stimulates Smad1 transcriptional activity, whereas phosphorylation by MAPK has the opposite effect. Neural tissue is formed only at very low levels of activity of BMP-transducing Smads, which require the combination of both low BMP levels and high MAPK signals. Many of the molecular players that regulate D-V patterning via regulation of BMP signaling have been conserved between Drosophila and the vertebrates.
Figures


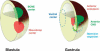
References
-
- Agathon A, Thisse C, Thisse B. The molecular nature of the zebrafish tail organizer. Nature. 2003;424:448–52. - PubMed
-
- Arendt D, Nubler-Jung K. Comparison of early nerve cord development in insects and vertebrates. Development. 1999;126:2309–25. - PubMed
-
- Aubert J, Dunstan H, Chambers I, Smith A. Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat. Biotechnol. 2002;20:1240–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

