Intracellular Ca2+ regulates the phosphorylation and the dephosphorylation of ciliary proteins via the NO pathway
- PMID: 15477378
- PMCID: PMC2234008
- DOI: 10.1085/jgp.200409153
Intracellular Ca2+ regulates the phosphorylation and the dephosphorylation of ciliary proteins via the NO pathway
Abstract
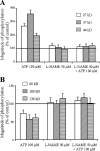
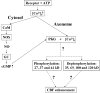
The phosphorylation profile of ciliary proteins under basal conditions and after stimulation by extracellular ATP was investigated in intact tissue and in isolated cilia from porcine airway epithelium using anti-phosphoserine and anti-phosphothreonine specific antibodies. In intact tissue, several polypeptides were serine phosphorylated in the absence of any treatment (control conditions). After stimulation by extracellular ATP, changes in the phosphorylation pattern were detected on seven ciliary polypeptides. Serine phosphorylation was enhanced for three polypeptides (27, 37, and 44 kD), while serine phosphorylation was reduced for four polypeptides (35, 69, 100, and 130 kD). Raising intracellular Ca2+ with ionomycin induced identical changes in the protein phosphorylation profile. Inhibition of the NO pathway by inhibiting either NO synthase (NOS), guanylyl cyclase (GC), or cGMP-dependent protein kinase (PKG) abolished the changes in phosphorylation induced by ATP. The presence of PKG within the axoneme was demonstrated using a specific antibody. In addition, in isolated permeabilized cilia, submicromolar concentrations of cGMP induced protein phosphorylation. Taken together, these results suggest that the axoneme is an integral part of the intracellular NO pathway. The surprising observation that ciliary activation is accompanied by sustained dephosphorylation of ciliary proteins via NO pathway was not detected in isolated cilia, suggesting that the protein phosphatases were either lost or deactivated during the isolation procedure. This work reveals that any pharmacological manipulation that abolished phosphorylation and dephosphorylation also abolished the enhancement of ciliary beating. Thus, part or all of the phosphorylated polypeptides are likely directly involved in axonemal regulation of ciliary beating.
Figures







Similar articles
-
The role of cGMP in the regulation of rabbit airway ciliary beat frequency.J Physiol. 2003 Sep 15;551(Pt 3):765-76. doi: 10.1113/jphysiol.2003.041707. Epub 2003 Jun 20. J Physiol. 2003. PMID: 12819300 Free PMC article.
-
Involvement of the nitric oxide-cyclic GMP pathway and neuronal nitric oxide synthase in ATP-induced Ca2+ signalling in cochlear inner hair cells.Eur J Neurosci. 2005 Jun;21(11):2912-22. doi: 10.1111/j.1460-9568.2005.04135.x. Eur J Neurosci. 2005. PMID: 15978003
-
Alcohol stimulates ciliary motility of isolated airway axonemes through a nitric oxide, cyclase, and cyclic nucleotide-dependent kinase mechanism.Alcohol Clin Exp Res. 2009 Apr;33(4):610-6. doi: 10.1111/j.1530-0277.2008.00875.x. Epub 2009 Jan 12. Alcohol Clin Exp Res. 2009. PMID: 19183138 Free PMC article.
-
Regulation of mammalian ciliary beating.Annu Rev Physiol. 2007;69:401-22. doi: 10.1146/annurev.physiol.69.040705.141253. Annu Rev Physiol. 2007. PMID: 16945069 Review.
-
Efficient mucociliary transport relies on efficient regulation of ciliary beating.Respir Physiol Neurobiol. 2008 Nov 30;163(1-3):202-7. doi: 10.1016/j.resp.2008.05.010. Epub 2008 May 22. Respir Physiol Neurobiol. 2008. PMID: 18586580 Review.
Cited by
-
Acquired cilia dysfunction in chronic rhinosinusitis.Am J Rhinol Allergy. 2012 Jan-Feb;26(1):1-6. doi: 10.2500/ajra.2012.26.3716. Am J Rhinol Allergy. 2012. PMID: 22391065 Free PMC article. Review.
-
Effects of cigarette smoke and alcohol on ciliated tracheal epithelium and inflammatory cell recruitment.Am J Respir Cell Mol Biol. 2007 Apr;36(4):452-9. doi: 10.1165/rcmb.2005-0440OC. Epub 2006 Nov 1. Am J Respir Cell Mol Biol. 2007. PMID: 17079783 Free PMC article.
-
Regulation of ciliary motility: conserved protein kinases and phosphatases are targeted and anchored in the ciliary axoneme.Arch Biochem Biophys. 2011 Jun 15;510(2):93-100. doi: 10.1016/j.abb.2011.04.003. Epub 2011 Apr 14. Arch Biochem Biophys. 2011. PMID: 21513695 Free PMC article. Review.
-
Ciliary beat frequency is maintained at a maximal rate in the small airways of mouse lung slices.Am J Respir Cell Mol Biol. 2006 Jul;35(1):110-7. doi: 10.1165/rcmb.2005-0417OC. Epub 2006 Feb 16. Am J Respir Cell Mol Biol. 2006. PMID: 16484686 Free PMC article.
-
Forces applied by cilia measured on explants from mucociliary tissue.Biophys J. 2007 Mar 1;92(5):1813-23. doi: 10.1529/biophysj.106.094698. Epub 2006 Dec 1. Biophys J. 2007. PMID: 17142280 Free PMC article.
References
-
- Bonini, N.M., and D.L. Nelson. 1990. Phosphoproteins associated with cyclic nucleotide stimulation of ciliary motility in Paramecium. J. Cell Sci. 95:219–230. - PubMed
-
- Bousquet, C., D.W. Ray, and S. Melmed. 1997. A common pro-opiomelanocortin-binding element mediates leukemia inhibitory factor and corticotropin-releasing hormone transcriptional synergy. J. Biol. Chem. 272:10551–10557. - PubMed
-
- Bracho, G.E., J.J. Fritch, and J.S. Tash. 1998. Identification of flagellar proteins that initiate the activation of sperm motility in vivo. Biochem. Biophys. Res. Commun. 242:231–237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

