Surgery and adjuvant dendritic cell-based tumour vaccination for patients with relapsed malignant glioma, a feasibility study
- PMID: 15477864
- PMCID: PMC2409960
- DOI: 10.1038/sj.bjc.6602195
Surgery and adjuvant dendritic cell-based tumour vaccination for patients with relapsed malignant glioma, a feasibility study
Abstract
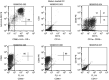
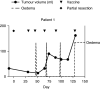
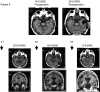
Patients with relapsed malignant glioma have a poor prognosis. We developed a strategy of vaccination using autologous mature dendritic cells loaded with autologous tumour homogenate. In total, 12 patients with a median age of 36 years (range: 11-78) were treated. All had relapsing malignant glioma. After surgery, vaccines were given at weeks 1 and 3, and later every 4 weeks. A median of 5 (range: 2-7) vaccines was given. There were no serious adverse events except in one patient with gross residual tumour prior to vaccination, who repetitively developed vaccine-related peritumoral oedema. Minor toxicities were recorded in four out of 12 patients. In six patients with postoperative residual tumour, vaccination induced one stable disease during 8 weeks, and one partial response. Two of six patients with complete resection are in CCR for 3 years. Tumour vaccination for patients with relapsed malignant glioma is feasible and likely beneficial for patients with minimal residual tumour burden.
Figures
References
-
- Black KL, Chen K, Becker DP, Merrill JE (1992) Inflammatory leukocytes associated with increase immunosuppression by glioblastoma. J Neurosurg 77: 120–126 - PubMed
-
- Brada M, Hoang-Xuan K, Rampling R, Dietrich PY, Dirix LY, Macdonald D, Heimans JJ, Zonnenberg BA, Bravo-Marques JM, Henriksson R, Stupp R, Yue N, Bruner J, Dugan M, Rao S, Zaknoen S (2001) Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol 12: 259–266 - PubMed
-
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 - PubMed
-
- Chang AE, Redman BG, Whitfield JR, Nickoloff BJ, Braun TM, Lee PP, Geiger JD, Mule JJ (2002) A phase I trial of tumor lysate-pulsed dendritic cells in the treatment of advanced cancer. Clin Cancer Res 8: 1021–1032 - PubMed
-
- Clay TM, Hobeika AC, Mosca PJ, Lyerly HK, Morse MA (2001) Assays for monitoring cellular immune responses to active immunotherapy of cancer. Clin Cancer Res 7: 1127–1135 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

