Prelamin A endoproteolytic processing in vitro by recombinant Zmpste24
- PMID: 15479156
- PMCID: PMC1134940
- DOI: 10.1042/BJ20041359
Prelamin A endoproteolytic processing in vitro by recombinant Zmpste24
Abstract
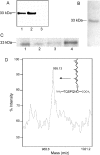
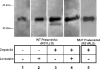
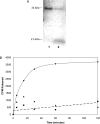
The nuclear lamins form a karyoskeleton providing structural rigidity to the nucleus. One member of the lamin family, lamin A, is first synthesized as a 74 kDa precursor, prelamin A. After the endopeptidase and methylation reactions which occur after farnesylation of the CAAX-box cysteine, there is a second endoproteolysis that occurs 15 amino acids upstream from the C-terminal farnesylated cysteine residue. Studies with knockout mice have implicated the enzyme Zmpste24 (Face-1) as a suitable candidate to perform one or both of these proteolytic reactions. Evidence has been presented elsewhere establishing that Zmpste24 possesses a zinc-dependent CAAX endopeptidase activity. In the present study, we confirm this CAAX endopeptidase activity with recombinant, membrane-reconstituted Zmpste24 and show that it can accept a prelamin A farnesylated tetrapeptide as substrate. To monitor the second upstream endoproteolytic cleavage of prelamin A, we expressed a 33 kDa prelamin A C-terminal tail in insect cells. We demonstrate that this purified substrate possesses a C-terminal farnesylated and carboxyl-methylated cysteine and, therefore, constitutes a valid substrate for assaying the second endoproteolytic step in lamin A maturation. With this substrate, we demonstrate that insect cell membranes bearing recombinant Zmpste24 can also catalyse the second upstream endoproteolytic cleavage.
Figures



References
-
- Glomset J. A., Gelb M. H., Farnsworth C. C. Prenyl proteins in eukaryotic cells: a new type of membrane anchor. Trends Biochem. Sci. 1990;15:139–142. - PubMed
-
- Rando R. R. Chemical biology of protein isoprenylation/methylation. Biochim. Biophys. Acta. 1996;1300:5–16. - PubMed
-
- Zhang F. L., Casey P. J. Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 1996;65:241–269. - PubMed
-
- Sinensky M. Recent advances in the study of prenylated proteins. Biochim. Biophys. Acta. 2000;1484:93–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

