PEG 3350 (Transipeg) versus lactulose in the treatment of childhood functional constipation: a double blind, randomised, controlled, multicentre trial
- PMID: 15479678
- PMCID: PMC1774276
- DOI: 10.1136/gut.2004.043620
PEG 3350 (Transipeg) versus lactulose in the treatment of childhood functional constipation: a double blind, randomised, controlled, multicentre trial
Abstract
Background: Recently, polyethylene glycol (PEG 3350) has been suggested as a good alternative laxative to lactulose as a treatment option in paediatric constipation. However, no large randomised controlled trials exist evaluating the efficacy of either laxative.
Aims: To compare PEG 3350 (Transipeg: polyethylene glycol with electrolytes) with lactulose in paediatric constipation and evaluate clinical efficacy/side effects.
Patients: One hundred patients (aged 6 months-15 years) with paediatric constipation were included in an eight week double blinded, randomised, controlled trial.
Methods: After faecal disimpaction, patients <6 years of age received PEG 3350 (2.95 g/sachet) or lactulose (6 g/sachet) while children > or =6 years started with 2 sachets/day. Primary outcome measures were: defecation and encopresis frequency/week and successful treatment after eight weeks. Success was defined as a defecation frequency > or =3/week and encopresis < or =1 every two weeks. Secondary outcome measures were side effects after eight weeks of treatment.
Results: A total of 91 patients (49 male) completed the study. A significant increase in defecation frequency (PEG 3350: 3 pre v 7 post treatment/week; lactulose: 3 pre v 6 post/week) and a significant decrease in encopresis frequency (PEG 3350: 10 pre v 3 post/week; lactulose: 8 pre v 3 post/week) was found in both groups (NS). However, success was significantly higher in the PEG group (56%) compared with the lactulose group (29%). PEG 3350 patients reported less abdominal pain, straining, and pain at defecation than children using lactulose. However, bad taste was reported significantly more often in the PEG group.
Conclusions: PEG 3350 (0.26 (0.11) g/kg), compared with lactulose (0.66 (0.32) g/kg), provided a higher success rate with fewer side effects. PEG 3350 should be the laxative of first choice in childhood constipation.
Figures
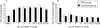
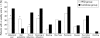
References
-
- Liptak GS, Baker SS, Colletti RB, et al. Constipation. In: Moyer VA, Elliot EJ, Davis RL, et al, eds. Evidence based paediatrics and child health. Manchester: BMJ Books 2000:264–72.
-
- Candelli M , Nista EC, Zocco MA, et al. Idiopathic chronic constipation: pathophysiology, diagnosis and treatment. Hepatogastroenterology 2001;48:1050–7. - PubMed
-
- Whitehead WE, Chaussade S, Corazziari E, et al. Report of an international workshop on management of constipation. Gastroenterol Int 1991;4:99–113.
-
- Schiller LR, Emmett M, Santa Ana CA, et al. Osmotic effects of polyethylene glycol. Gastroenterology 1988;94:933–41. - PubMed
-
- Brady CE III, DiPalma JA, Morawski SG, et al. Urinary excretion of polyethylene glycol 3350 and sulfate after gut lavage with a polyethylene glycol electrolyte lavage solution. Gastroenterology 1986;90:1914–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
