Antidiabetic thiazolidinediones inhibit invasiveness of pancreatic cancer cells via PPARgamma independent mechanisms
- PMID: 15479693
- PMCID: PMC1774261
- DOI: 10.1136/gut.2003.031997
Antidiabetic thiazolidinediones inhibit invasiveness of pancreatic cancer cells via PPARgamma independent mechanisms
Abstract
Background/aims: Thiazolidinediones (TZD) are a new class of oral antidiabetic drugs that have been shown to inhibit growth of some epithelial cancer cells. Although TZD were found to be ligands for peroxisome proliferators activated receptor gamma (PPARgamma) the mechanism by which TZD exert their anticancer effect is currently unclear. Furthermore, the effect of TZD on local motility and metastatic potential of cancer cells is unknown. The authors analysed the effects of two TZD, rosiglitazone and pioglitazone, on invasiveness of human pancreatic carcinoma cell lines in order to evaluate the potential therapeutic use of these drugs in pancreatic adenocarcinoma.
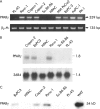
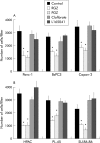
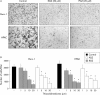
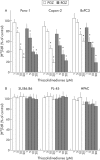
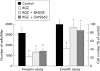
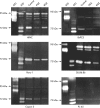
Methods: Expression of PPARgamma in human pancreatic adenocarcinomas and pancreatic carcinoma cell lines was measured by reverse transcription polymerase chain reaction and confirmed by western blot analysis. PPARgamma activity was evaluated by transient reporter gene assay. Invasion assay was performed in modified Boyden chambers. Gelatinolytic and fibrinolytic activity were evaluated by gel zymography.
Results: TZD inhibited pancreatic cancer cells' invasiveness, affecting gelatinolytic and fibrinolytic activity with a mechanism independent of PPARgamma activation and involving MMP-2 and PAI-1 expression.
Conclusion: TZD treatment in pancreatic cancer cells has potent inhibitory effects on growth and invasiveness suggesting that these drugs may have application for prevention and treatment of pancreatic cancer in humans.
Figures

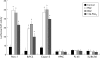



References
-
- Silverberg E , Boring CC, Squires TS. Cancer statistics 1990. Can Cancer J Clinitians 1990;40:9–26. - PubMed
-
- Hohne MW, Halatsch ME, Kahl GF, et al. Frequent loss of expression of the potential tumor suppressor gene DCC in ductal pancreatic adenocarcinoma. Cancer Res 1992;52:2616–19. - PubMed
-
- Bakkevold KE, Arnesjo B, Kambestang B. Carcinoma of the pancreas and Papilla of Vater: presenting symptoms, signs, and diagnosis related to stage and tumor site. A prospective multicenter trial in 472 patients. Scand J Gastroenterol 1992;27:317–25. - PubMed
-
- Rocha Lima CM, Centeno B. Update on pancreatic cancer. Curr Opin Oncol 2002;14:424–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
