Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors
- PMID: 15479739
- PMCID: PMC2172522
- DOI: 10.1083/jcb.200404068
Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors
Abstract
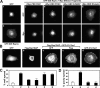
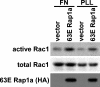
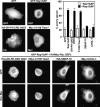
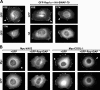
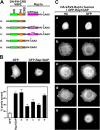
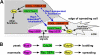
The Ras-related GTPase Rap1 stimulates integrin-mediated adhesion and spreading in various mammalian cell types. Here, we demonstrate that Rap1 regulates cell spreading by localizing guanine nucleotide exchange factors (GEFs) that act via the Rho family GTPase Rac1. Rap1a activates Rac1 and requires Rac1 to enhance spreading, whereas Rac1 induces spreading independently of Rap1. Active Rap1a binds to a subset of Rac GEFs, including VAV2 and Tiam1 but not others such as SWAP-70 or COOL-1. Overexpressed VAV2 and Tiam1 specifically require Rap1 to promote spreading, even though Rac1 is activated independently of Rap1. Rap1 is necessary for the accumulation of VAV2 in membrane protrusions at the cell periphery. In addition, if VAV2 is artificially localized to the cell edge with the subcellular targeting domain of Rap1a, it increases cell spreading independently of Rap1. These results lead us to propose that Rap1 promotes cell spreading by localizing a subset of Rac GEFs to sites of active lamellipodia extension.
Figures

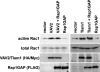

References
-
- Ardouin, L., M. Bracke, A. Mathiot, S.N. Pagakis, T. Norton, N. Hogg, and V.L. Tybulewicz. 2003. Vav1 transduces TCR signals required for LFA-1 function and cell polarization at the immunological synapse. Eur. J. Immunol. 33:790–797. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

