Influence of acylation sites of influenza B virus hemagglutinin on fusion pore formation and dilation
- PMID: 15479794
- PMCID: PMC523265
- DOI: 10.1128/JVI.78.21.11536-11543.2004
Influence of acylation sites of influenza B virus hemagglutinin on fusion pore formation and dilation
Abstract
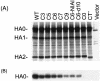
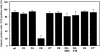
The cytoplasmic tail (CT) of hemagglutinin (HA) of influenza B virus (BHA) contains at positions 578 and 581 two highly conserved cysteine residues (Cys578 and Cys581) that are modified with palmitic acid (PA) through a thioester linkage. To investigate the role of PA in the fusion activity of BHA, site-specific mutagenesis was performed with influenza B virus B/Kanagawa/73 HA cDNA. All of the HA mutants were expressed on Cos cells by an expression vector. The membrane fusion ability of the HA mutants at a low pH was quantitatively examined with lipid (octadecyl rhodamine B chloride) and aqueous (calcein) dye transfer assays and with the syncytium formation assay. Two deacylation mutants lacking a CT or carrying serine residues substituting for Cys578 and Cys581 promoted full fusion. However, one of the single-acylation-site mutants, C6, in which Cys581 is replaced with serine, promoted hemifusion but not pore formation. In contrast, four other single-acylation-site mutants that have a sole cysteine residue in the CT at position 575, 577, 579, or 581 promoted full fusion. The impaired pore-forming ability of C6 was improved by amino acid substitution between residues 578 and 582 or by deletion of the carboxy-terminal leucine at position 582. Syncytium-forming ability, however, was not adequately restored by these mutations. These facts indicated that the acylation was not significant in membrane fusion by BHA but that pore formation and pore dilation were appreciably affected by the particular amino acid sequence of the CT and the existence of a single acylation site in CT residue 578.
Figures




References
-
- Bullough, P. A., F. M. Hughson, J. J. Skehel, and D. C. Wiley. 1994. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature 371:37-43. - PubMed
-
- Carr, C. M., and P. S. Kim. 1993. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell 73:823-832. - PubMed
-
- Fischer, C., B. Schroth-Diez, A. Herrmann, W. Garten, and H. D. Klenk. 1998. Acylation of the influenza hemagglutinin modulates fusion activity. Virology 248:284-294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

