The varicella-zoster virus open reading frame 63 latency-associated protein is critical for establishment of latency
- PMID: 15479825
- PMCID: PMC523280
- DOI: 10.1128/JVI.78.21.11833-11840.2004
The varicella-zoster virus open reading frame 63 latency-associated protein is critical for establishment of latency
Abstract
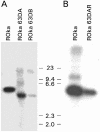
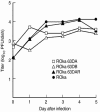
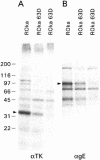
Varicella-zoster virus (VZV) expresses at least six viral transcripts during latency. One of these transcripts, derived from open reading frame 63 (ORF63), is one of the most abundant viral RNAs expressed during latency. The VZV ORF63 protein has been detected in human and experimentally infected rodent ganglia by several laboratories. We have deleted >90% of both copies of the ORF63 gene from the VZV genome. Animals inoculated with the ORF63 mutant virus had lower mean copy numbers of latent VZV genomes in the dorsal root ganglia 5 to 6 weeks after infection than animals inoculated with parental or rescued virus, and the frequency of latently infected animals was significantly lower in animals infected with the ORF63 mutant virus than in animals inoculated with parental or rescued virus. In contrast, the frequency of animals latently infected with viral mutants in other genes that are equally or more impaired for replication in vitro, compared with the ORF63 mutant, is similar to that of animals latently infected with parental VZV. Examination of dorsal root ganglia 3 days after infection showed high levels of VZV DNA in animals infected with either ORF63 mutant or parental virus; however, by days 6 and 10 after infection, the level of viral DNA in animals infected with the ORF63 mutant was significantly lower than that in animals infected with parental virus. Thus, ORF63 is not required for VZV to enter ganglia but is the first VZV gene shown to be critical for establishment of latency. Since the present vaccine can reactivate and cause shingles, a VZV vaccine based on the ORF63 mutant virus might be safer.
Figures




References
-
- Bontems, S., E. Di Valentin, L. Baudoux, B. Rentier, C. Sadzot-Delvaux, and J. Piette. 2002. Phosphorylation of varicella-zoster virus IE63 protein by casein kinase influences its cellular localization and gene regulation activity. J. Biol. Chem. 277:21050-21060. - PubMed
-
- Brunell, P. A., L. C. Ren, J. I. Cohen, and S. E. Straus. 1999. Viral gene expression in rat trigeminal ganglia following neonatal infection with varicella-zoster virus. J. Med. Virol. 58:286-290. - PubMed
-
- Davison, A. J., and J. Scott. 1986. The complete DNA sequence of varicella-zoster virus. J. Gen. Virol. 67:1759-1816. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

