Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies
- PMID: 15479838
- PMCID: PMC523246
- DOI: 10.1128/JVI.78.21.11980-11987.2004
Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies
Abstract
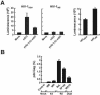
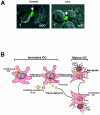
The tropism of human immunodeficiency virus type 1 for chemokine receptors plays an important role in the transmission of AIDS. Although CXCR4-tropic virus is more cytopathic for T cells, CCR5-tropic strains are transmitted more frequently in humans for reasons that are not understood. Phenotypically immature myeloid dendritic cells (mDCs) are preferentially infected by CCR5-tropic virus, in contrast to mature mDCs, which are not susceptible to infection but instead internalize virus into a protected intracellular compartment and enhance the infection of T cells. Here, we define a mechanism to explain preferential transmission of CCR5-tropic viruses based on their interaction with mDCs and sensitivity to neutralizing antibodies. Infected immature mDCs differentiated normally and were found to enhance CCR5-tropic but not CXCR4-tropic virus infection of T cells even in the continuous presence of neutralizing antibodies. Infectious synapses also formed normally in the presence of such antibodies. Infection of immature mDCs by CCR5-tropic virus can therefore establish a pool of infected cells that can efficiently transfer virus at the same time that they protect virus from antibody neutralization. This property of DCs may enhance infection, contribute to immune evasion, and could provide a selective advantage for CCR5-tropic virus transmission.
Figures
References
-
- Abrahamsen, T. G., C. S. Carter, E. J. Read, M. Rubin, H. G. Goetzman, E. F. Lizzio, Y. L. Lee, P. A. Pizzo, and T. Hoffman. 1991. Stimulatory effect of counterflow centrifugal elutriation in large-scale separation of peripheral blood monocytes can be reversed by storing the cells at 37°C. J. Clin. Apheresis 6:48-53. - PubMed
-
- Ayehunie, S., E. A. Garcia-Zepeda, J. A. Hoxie, R. Horuk, T. S. Kupper, A. D. Luster, and R. M. Ruprecht. 1997. Human immunodeficiency virus-1 entry into purified blood dendritic cells through CC and CXC chemokine coreceptors. Blood 90:1379-1386. - PubMed
-
- Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

