Mink epithelial cell killing by pathogenic murine leukemia viruses involves endoplasmic reticulum stress
- PMID: 15479849
- PMCID: PMC523263
- DOI: 10.1128/JVI.78.21.12071-12074.2004
Mink epithelial cell killing by pathogenic murine leukemia viruses involves endoplasmic reticulum stress
Abstract
We previously demonstrated that mink cells undergo apoptosis after MCF13 murine leukemia virus (MLV) infection. In this study, we observed that virus-infected mink epithelial cells had significantly larger amounts of steady-state levels of MCF13 MLV envelope precursor protein (gPr80(env)) than did Mus dunni fibroblasts, which are resistant to virus-induced cytopathicity. Infection of mink cells with the noncytopathic NZB-9 MLV did not result in the accumulation of gPr80(env). MCF13 MLV infection of mink cells produced low cell surface expression of envelope glycoprotein and less efficient spread of infectious virus. Western blot analysis of mink epithelial cells infected with MCF13 MLV showed an increase in GRP78/BiP, which was not observed for either mink cells infected with NZB-9 MLV or M. dunni fibroblasts infected with MCF13 MLV. MCF13 MLV infection of mink cells also resulted in a significant upregulation of CHOP/GADD153. These results indicate that the accumulation of MCF13 MLV gPr80(env) triggers endoplasmic reticulum stress, which may mediate apoptosis in mink epithelial cells.
Figures
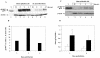
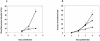


References
-
- Chattopadhyay, S. K., M. W. Cloyd, D. L. Linemeyer, M. R. Lander, E. Rands, and D. R. Lowy. 1982. Cellular origin and role of mink cell focus-forming viruses in murine thymic lymphomas. Nature 295:25-31. - PubMed
-
- Einfeld, D. 1996. Maturation and assembly of retroviral glycoproteins. Curr. Top. Microbiol. Immunol. 214:133-176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

